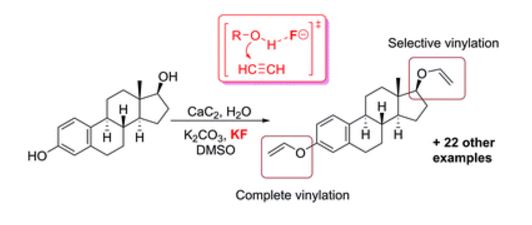
N.G. Voron’ko, S.R. Derkach, M.A. Vovk, P.M. Tolstoy
“Complexation of κ-carrageenan with gelatin in the aqueous phase analysed by 1H NMR kinetics and relaxation”
Carbohydrate Polymers, 2017, 169, 117–126
DOI: 10.1016/j.carbpol.2017.04.010

The 1H NMR spectroscopy is used to study the kinetics of gelation in the aqueous mixtures of κ-carrageenan with gelatin. The time dependence of NMR signals intensities shows that the kinetics of gel formation consists of classical ‘fast’ (rate constant k ≈ 6 h−1) and ‘slow’ (k ≈ 1 h−1) periods, corresponding to a coil → helix transition and subsequent aggregation of helices. Upon increase of the κ-carrageenan/gelatin (w/w) ratio Z the rate of the fast process slows down by a factor of 1.6–2.4. Further analysis was done by studying the dependence of spin-spin relaxation times of protons of gelatin on Z in the aqueous phase. A qualitative scheme describing hydrogel formation in the complex solution is given. It is hypothesized that at higher concentration of PECs the hydrogel structure network is stabilized by three types of nodes: triple helices of gelatin and intra-/inter-molecular double helices of κ-carrageenan.