Mikhail A. Kinzhalov, Konstantin V. Luzyanin, Irina A. Boyarskaya, Galina L. Starova, Vadim P. Boyarskiy
“Synthetic and structural investigation of [PdBr2(CNR)2] (R = Cy, Xyl)”
Journal of Molecular Structure 2014, 1068, 222–227
DOI: 10.1016/j.molstruc.2014.04.025

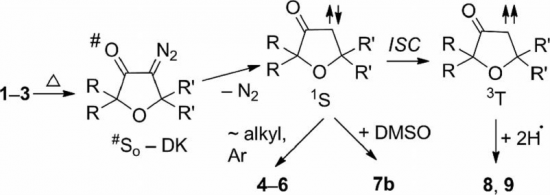
Reaction of cis-dichloro-bis-(cyclohexylisocyanide)palladium(II) ([PdCl2(CNCy)2], (1) or cis-dichloro-bis-(2,6-dimethylphenyl-isocyanide)palladium(II) ([PdCl2(CNXyl)2], (2) with excess of potassium bromide in acetone furnished complexes trans-dibromo-bis-(cyclohexylisocyanide)palladium(II) ([PdBr2(CNCy)2], (3) or trans-dibromo-bis-(2,6-dimethylphenylisocyanide)palladium(II) ([PdBr2(CNXyl)2], (4), respectively. Both compounds were characterized by elemental analyses (C, H, N), high resolution ESI+-MS, IR, 1H and 13C{1H} NMR spectroscopies, and their crystals were analyzed by a single-crystal and powder X-ray diffraction methods. To rationalise the dependence of the structure of 1–4 on type of halogen ligands, the Gibbs free energies of corresponding cis- and trans-isomers of 1–4 were estimated at the DFT B3LYP level of theory.