N.G. Voron’ko, S.R. Derkach, M.A. Vovk, P.M. Tolstoy
“Formation of -carrageenan–gelatin polyelectrolyte complexes studied by 1H NMR, UV spectroscopy and kinematic viscosity measurements”
Carbohydrate Polymers, 2016, 151, 1152-1161
DOI:10.1016/j.carbpol.2016.06.060
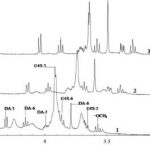
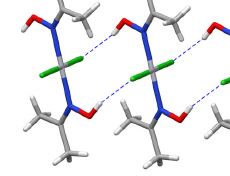
The intermolecular interactions between an anionic polysaccharide from the red algae κ-carrageenan and a gelatin polypeptide, forming stoichiometric polysaccharide–polypeptide (bio)polyelectrolyte complexes in the aqueous phase, were examined. The major method of investigation was high-resolution 1H NMR spectroscopy. Additional data were obtained by UV absorption spectroscopy, light scattering dispersion and capillary viscometry. Experimental data were interpreted in terms of the changing roles of electrostatic interactions, hydrophobic interactions and hydrogen bonds when κ-carrageenan–gelatin complexes are formed. At high temperatures, when biopolymer macromolecules in solution are in the state of random coil, hydrophobic interactions make a major contribution to complex stabilization. At the temperature of gelatin’s coil → helix conformational transition and at lower temperatures, electrostatic interactions and hydrogen bonds play a defining role in complex formation. A proposed model of the κ-carrageenan–gelatin complex is discussed.