CMR was visited by participants Nationwide chemical tournament for schoolchildren.
Archive for March 30, 2016
NMR Without Pulses
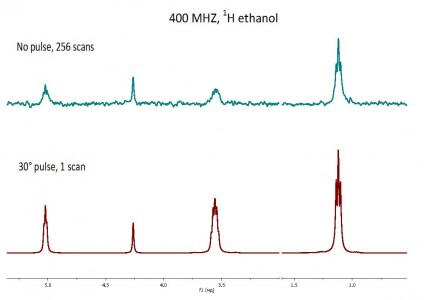
We have attempted to reproduce the Glenn’s experiment of observation of spontaneous excitation and relaxation in magnetic field for ethanol. In the picture above shows the results of adding 256 magnitude spectra obtained without RF pulse (top trace) and spectrum of ethanol collected with one scan using a 30° pulse (bottom trace).
J. Wood Chem. Technol. 2016, 36, 259-269
E.I. Evstigneyev, O.S. Yuzikhin, A.A. Gurinov, A.Yu. Ivanov, T.O. Artamonova, M.A. Khodorkovskiy, E.A. Bessonova, A.V. Vasilyev
“Study of Structure of Industrial Acid Hydrolysis Lignin, Oxidized in the H2O2-H2SO4 System”
J. Wood Chem. Technol., 2016, 36, 259-269
DOI:10.1080/02773813.2015.1137945
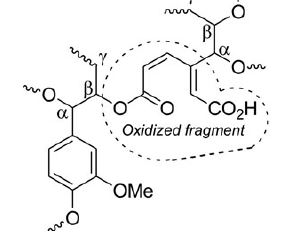
Products of oxidation of industrial acid hydrolysis lignin in the H2O2-H2SO4 system were studied using 13C NMR (in solution and solid state), MALDI-MS, and MS(ESI) techniques. Oxidation of hydrolysis lignin leads to the opening of aromatic rings of lignin, yielding carboxylic groups. Alkyl aryl ether linkages (β-O-4-bonds) between lignin phenyl propane units are not significantly affected by the oxidation. The structure of oxidized hydrolysis lignin is proposed. The basic structural unit of oxidized hydrolysis lignin is a muconic acid derivative.
NMR Without Pulses
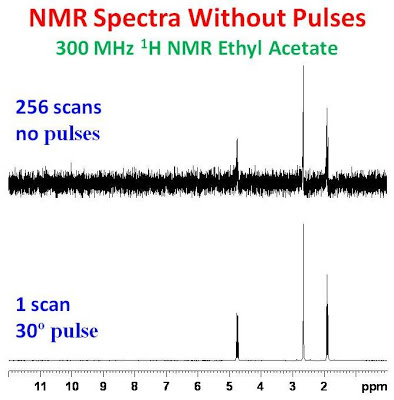
Glenn Facey (University of Ottawa) introduced interesting results observation of spontaneous excitation and relaxation for samples in magnetic field. Experiment was conducted without rf pulses and FIDs were collected apartly. The spectrum plotted is the result of adding 256 magnitude spectra.
Source: University of Ottawa NMR Facility Blog
Electrolytic Cell for EPR
New additional equipment Electrolytic Cell WG-810-Q for Bruker Elexsys E580 is available now (potentiostat/galvanostat is required for conducting experiments).
Inorg. Chem. 2016
V.V. Sivchik, E.V. Grachova, A.S. Melnikov, S.N. Smirnov, A.Yu. Ivanov, P.Hirva, S.P. Tunik, I.O. Koshevoy
“Solid-State and Solution Metallophilic Aggregation of a Cationic [Pt(NCN)L]+ Cyclometalated Complex”
Inorg. Chem., 2016, ASAP
DOI:10.1021/acs.inorgchem.5b02713
The noncovalent intermolecular interactions (π–π stacking, metallophilic bonding) of the cyclometalated complexes [Pt(NCN)L]+X– (NCN = dipyridylbenzene, L = pyridine (1), acetonitrile (2)) are determined by the steric properties of the ancillary ligands L in the solid state and in solution, while the nature of the counterion X– (X– = PF6–, ClO4–, CF3SO3–) affects the molecular arrangement of 2·X in the crystal medium. According to the variable-temperature X-ray diffraction measurements, the extensive Pt···Pt interactions and π-stacking in 2·X are significantly temperature-dependent. The variable concentration 1H and diffusion coefficients NMR measurements reveal that 2·X exists in the monomeric form in dilute solutions at 298 K, while upon increase in concentration [Pt(NCN)(NCMe)]+ cations undergo the formation of the ground-state oligomeric aggregates with an average aggregation number of ∼3. The photoluminescent characteristics of 1 and 2·X are largely determined by the intermolecular aggregation. For the discrete molecules the emission properties are assigned to metal perturbed IL charge transfer mixed with some MLCT contribution. In the case of oligomers 2·X the luminescence is significantly red-shifted with respect to 1 and originates mainly from the 3MMLCT excited states. The emission energies depend on the structural arrangement in the crystal and on the complex concentration in solution, variation of which allows for the modulation of the emission color from greenish to deep red. In the solid state the lability of the ligands L leads to vapor-induced reversible transformation 1 ↔ 2 that is accompanied by the molecular reorganization and, consequently, dramatic change of the photophysical properties. Time-dependent density functional theory calculations adequately support the models proposed for the rationalization of the experimental observations.
Open Doors Day
Organic Lett. 2015, 17, 3930-3933
D. Dar’In, O. Bakulina, M. Chizhova, M. Krasavin
“New Heterocyclic Product Space for the Castagnoli-Cushman Three-Component Reaction”
Organic Lett., 2015, 17, 3930-3933
DOI:10.1021/acs.orglett.5b02014
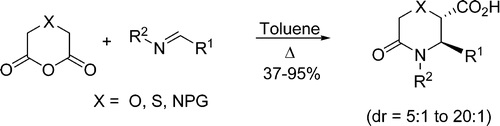
Significant expansion of heterocyclic product space accessible by the Castagnoli–Cushman reaction (CCR) has been achieved via the use of glutaric anhydride analogues containing endocyclic substitutions with oxygen, nitrogen, and sulfur. Incorporation of these heteroatoms in the anhydride’s backbone results in enhanced reactivity and generally lower temperatures that are required for the reactions to go to completion. These findings are particularly significant in light of the CCR recently recognized as an efficient tool for lead-oriented synthesis.
Excursion-lecture for “LETI” students

Excursion-lecturer was conducted in the Center for Magnetic Resonance for Saint-Petersburg Electrotechnical University (“LETI”) students. Equipment for modern NMR techniques were demonstrated by Mikhail Vovk, demonstration of operation on the EPR spectrometer Bruker Elexsys E580 was conducted by Stanislav Sukharzhevskii.
J. Org. Chem., 2016, 81, 1967-1980
S. Saulnier, A.A. Golovanov, A.Yu. Ivanov, I.A. Boyarskaya, A.V. Vasilyev
“Transformations of Conjugated Enynones in the Superacid CF3SO3H. Synthesis of Butadienyl Triflates, Indanones, and Indenes”
J. Org. Chem., 2016, 81, 1967-1980
DOI:10.1021/acs.joc.5b02785
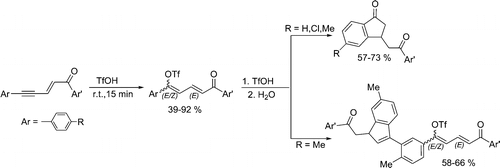
Conjugated 1,5-diarylpent-2-en-4-yn-1-ones add the superacid CF3SO3H to the acetylenic bond with formation of the corresponding butadienyl triflates. Under superacidic reaction conditions, these triflates are transformed into indanone or indene derivatives depending on which substituents on the aromatic ring are conjugated with the butadiene fragment. In a less acidic system (10% vol pyridine in CF3SO3H) only the formation of butadienyl triflates takes place. Cationic reaction intermediates were studied by means of NMR and DFT calculations.