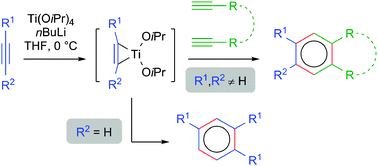
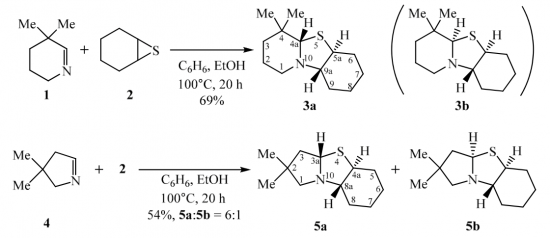
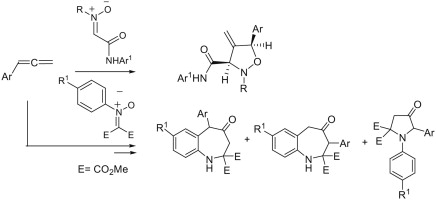
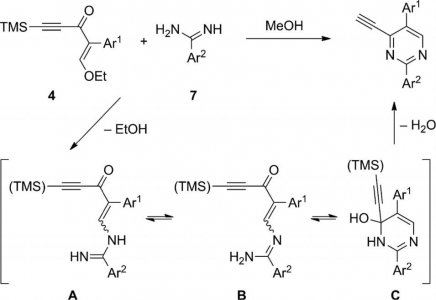
A delegation of the Hannover Medical School (Medizinische Hochschule Hannover) has visited our Center today:
- Dr. Tilman Fabian, Management Cluster of Excellence “Rebirth”, expert in management of interdisciplinary research projects
- Prof. Dr. Ullrich Martin, Leibniz Research Laboratory for Biotechnology and Artificial Organs, expert in iPS cells
- Prof. Dr. Denise Hilfiker-Kleiner, Dean of Research, Hannover Medical School, expert in experimental Cardiology
- Dr. Robert Zweigerdt, Leibniz Research Laboratory for Biotechnology and Artificial Organs, expert in cell mass production/bioreactors
- Dr. Andres Hilfiker, Leibniz Research Laboratory for Biotechnology and Artificial Organs, expert in Tissue Engineering
- Prof. Dr. Axel Schambach, PhD, Department of Hematology, Hannover Medical School, expert in Experimental Hematology
- Prof. Dr. Alexander Heisterkamp, Leibniz University Hannover, Hannover Laser Center, expert in Biophotonics