A.N. Shestakov, A.S. Pankova, P. Golubev, A.F. Khlebnikov, M.A. Kuznetsov
“Brønsted acid mediated cyclizations of ortho-aryl(ethynyl)pyrimidines”
Tetrahedron, 2017, 73 (27-28), 3939–3948
DOI: 10.1016/j.tet.2017.05.070
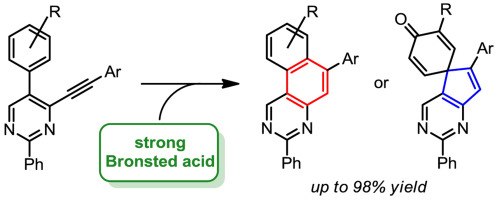
A high-yielding procedure for the synthesis of 5-aryl-4-(arylethynyl)pyrimidines from easily available 2-aryl-3-hydroxyacrylates is reported. These pyrimidines readily undergo cyclization in strong Brønsted acids and, depending on the substitution in alkynylpyrimidines and the water content of the reaction mixture, yield either benzo[f]quinazolines or derivatives of spiro[cyclohexa-2,5-diene-1,5′-cyclopenta[d]pyrimidin]-4-one. In most cases the cyclization proceeds nearly quantitatively. DFT calculations support the proposed mechanisms induced by the protonation of the triple bond in 5-aryl-4-(arylethynyl)pyrimidines. Fluorescent properties of the obtained heterocycles are also described.