D.A. Markelov, V.V. Matveev, P. Ingman, M.N. Nikolaeva, A.V. Penkova, E. Lahderanta, N.I. Boiko, V.I. Chizhik
“Unexpected Temperature Behavior of Polyethylene Glycol Spacers in Copolymer Dendrimers in Chloroform”
Sci. Rep., 2016, 6, 24270
DOI:10.1038/srep24270
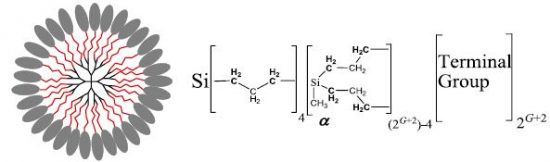
We have studied copolymer dendrimer structure: carbosilane dendrimers with terminal phenylbenzoate mesogenic groups attached by poly(ethylene) glycol (PEG) spacers. In this system PEG spacers are additional tuning to usual copolymer structure: dendrimer with terminal mesogenic groups. The dendrimer macromolecules were investigated in a dilute chloroform solution by 1H NMR methods (spectra and relaxations). It was found that the PEG layer in G = 5 generations dendrimer is “frozen” at high temperatures (above 260 K), but it unexpectedly becomes “unfrozen” at temperatures below 250 K (i.e., melting when cooling). The transition between these two states occurs within a small temperature range (~10 K). Such a behavior is not observed for smaller dendrimer generations (G = 1 and 3). This effect is likely related to the low critical solution temperature (LCST) of PEG and is caused by dendrimer conformations, in which the PEG group concentration in the layer increases with growing G. We suppose that the unusual behavior of PEG fragments in dendrimers will be interesting for practical applications such as nanocontainers or nanoreactors.