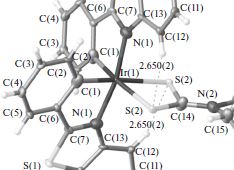
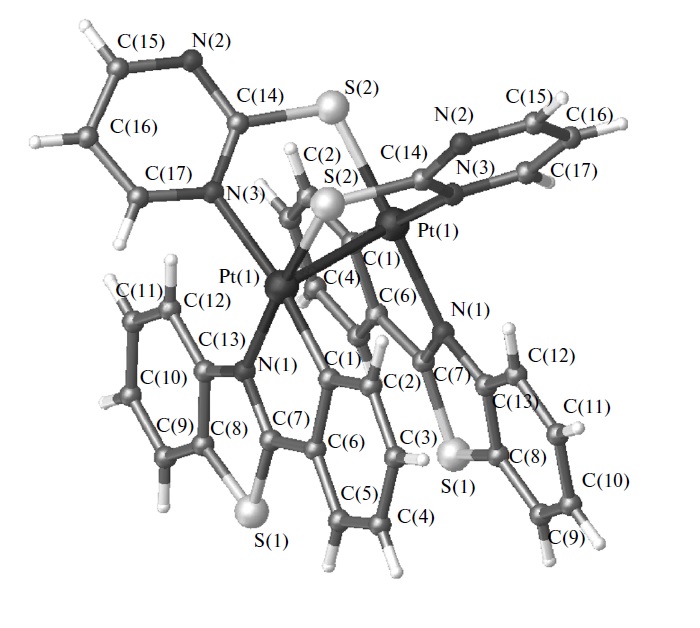
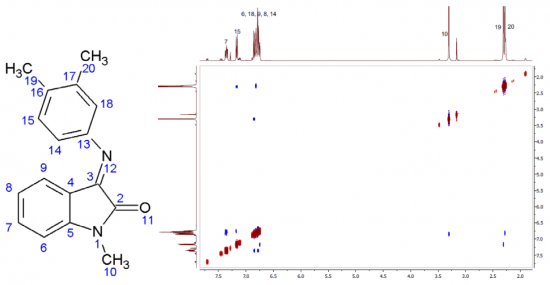
A.S. Smirnov, D.N. Nikolaev, V.V. Gurzhiy, S.N. Smirnov, V.S. Suslonov, A.V. Garabadzhiu, P.B. Davidovich
“Conformational stabilization of isatin Schiff bases – biologically active chemical probes”
RSC Adv., 2017, 7, 10070-10073
DOI: 10.1039/c6ra26779c
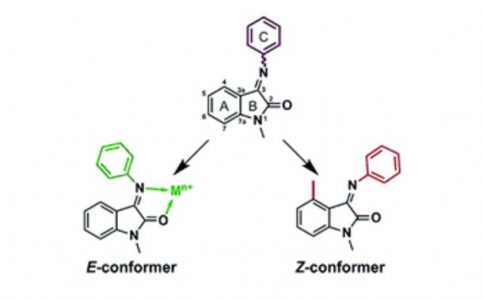
Isatin Schiff base derivatives have a wide range of biological effects. Unfortunately, these compounds possess a serious topological shortcoming: the conformational E ⇆ Z interconversion. Two ways of conformation stabilization are reported here: complexation with metals that stabilize the E-conformer and substitution in the 4th position of the isatin core stabilizing the Z-form.