CMR specialist Alexander Ivanov held four sessions with bachelor students of chemical faculty dedicated to NMR spectroscopy technique, sample preparation and analysis of the spectra.
Archive for March 31, 2014
Educational tasks
Pupils visit
On 27th of March at 15:30 in the CMR will be given a short tour for 200 students of the Lyceum of Novosibirsk.
On 27th of March at 15:30 in the CMR will be given a short tour for participants of XXXVIII Russian Chemistry Conference for pupils.
Interesting for 21st of March
At 15:30 in the auditorium 2076 of Chemistry Faculty will be a lecture by Professor Miklos Gratzl “Optochemical and electrochemical methods for biomedical measurements” (in addition to previously announced talk of Prof. Gratzl on 20 of March at CMR).
At 17:00 in the auditorium 404 of Institute of Physics (building M) will be a seminar on physical models of birds navigation magnetoreception. Program:
- Lecture “Introduction to MRI” by K.V. Tyutyukin. In this talk basics of the method and its in vivo applications will be given.
- A. V. Komolkin will review some publications on MRI in birds investigation.
Congratulations

CMR specialist Svetlana Pylaeva, 2nd year Master student, took this semester third place in the ranking of all students of physical faculty.
Open Day on April 2
On Wednesday, April 2, 2014 during the conference “Mendeleev-2014” there will take place an open day in our Center. A tour around the Center is scheduled to start at 15:00 to 16:30 for all interested conference participants.
Tetrahedron Letters 2014
Alena S. Pankova, Mikhail A. Kuznetsov
“Synthesis and thermal transformations of spiro-fused N-phthalimidoaziridines”
Tetrahedron Letters, 2014, In Press
DOI: 10.1016/j.tetlet.2014.03.014
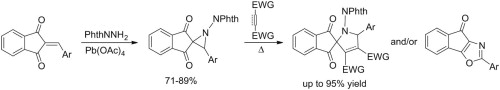
Oxidation of N-aminophthalimide in the presence of 2-arylideneinden-1,3-diones with electron-withdrawing substituents gives the corresponding 3-aryl-1-phthalimidospiro[aziridine-2,2’-indene]-1’,3’-diones in good yields. Heating these aziridines with standard dipolarophiles (N-phenylmaleimide, dimethyl acetylenedicarboxylate, maleate and fumarate) leads, in most cases, to spiro[inden-2,2’-pyrrole] derivatives as products of 1,3-dipolar cycloaddition of the intermediate azomethine ylides with up to 70-95% yields in the case of N-phenylmaleimide. As is typical for 2-acylaziridines, the competing rearrangement into 2-aryl-4H-indeno[2,1-d][1,3]oxazol-4-ones prevails for less active dipolarophiles. Increasing the electron-releasing properties of the 3-aryl ring allows the observation of the push-pull effect of electron-donating and electron-withdrawing substituents on the ease of the three-membered ring-opening.
The first lecture of Prof. Gratzl
Prof. Miklos Gratzl gave his first lecture of the two planned. The second lecture will be held a week later, on March 20.
Announcement of lectures was here.
Visiting rectors
Representatives of the Council of Rectors of the North-West Region have visited our Center with an introductory tour. The guests got acquainted with the equipment and learned the principles of Center’s work.
News on the site of the University.
Chemistry of Heterocyclic Compounds
M. A. Kuznetsov, A. Ya. Bespalov
“One-pot, Three-component Synthesis of [1,3]thiazolo[4,3-b][1,3,4]thiadiazoles: Correct Structure of the Products”
Chem. Heterocycl. Compd., 2014, 49, 1458-1463
DOI: 10.1007/s10593-014-1396-4
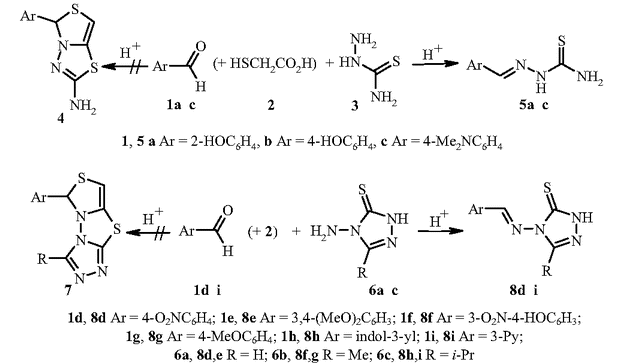
The products of the one-pot, three-component synthesis of [1,3]thiazolo-[4,3-b][1, 3, 4]thiadiazoles from aromatic aldehydes, thioglycolic acid, and compounds containing a C(=S)–N–NH2 fragment (thiosemicarbazide or 4-amino-2,4-dihydro-3H-1,2,4-triazole-3-thiones) are not condensed hetero-cycles (as reported by several researchers), but are thiosemicarbazones or triazolylimines of the aldehydes used.
Dalton Transactions
Julia R. Shakirova, Elena V. Grachova, Antti J. Karttunen, Vladislav V. Gurzhiy, Sergey P. Tunik and Igor O. Koshevoy
“Metallophilicity-assisted assembly of phosphine-based cage molecules”
Dalton Trans., 2014, Advance Article
DOI: 10.1039/c3dt53645a
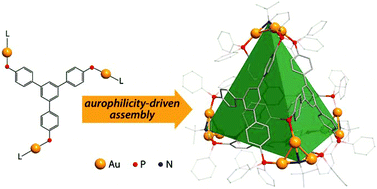
A family of supramolecular cage molecules has been obtained via self-assembly of the phosphine–gold coordination complexes following an aurophilicity-driven aggregation approach. Use of the di- (PP) or tridentate (PPP) phosphine ligands Pn (n = 2, 3) with rigid polyaromatic backbones leads to clean formation of the coordination Pn(Au(tht))nn+ species, sequential treatment of which with H2O/NEt3 and excess of H2NBut gives the finite 3D structures of two major types. The cylindrical-like hexametallic cages [(PPAu2)3(μ3-NBut)2]2+ are based on the diphosphines PP = 1,4-bis(diphenylphosphino)benzene (1), 4,4′-bis(diphenylphosphino)biphenyl (2), 4,4′′-bis(diphenylphosphino)terphenyl (3), while the triphosphine PPP (1,3,5-tris(diphenylphosphinophenyl)benzene) produces a tetrahedral dodecagold complex [(PPPAu3)4(μ3-NBut)4]4+ (4). The cages 1–4 have been studied using the ESI-MS and 1H, 31P NMR spectroscopy, and the crystal structures of 1 and 4 were determined by an X-ray diffraction study. The NMR spectroscopic investigations showed that cylindrical complexes 1–3 undergo twisting-like interconversion of the helical P↔M isomers in solution, while 4 is a stereochemically rigid compound retaining its axially chiral architecture. The difference in dynamic behaviour was rationalized using computational studies with density functional methods.