J.J. Medvedev, O.S. Galkina, A.A. Klinkova, D.S. Giera, L. Hennig, C. Schneider, V.A. Nikolaev
“Domino [4 + 1]-annulation of α,β-unsaturated δ-amino esters with Rh(II)–carbenoids – a new approach towards multi-functionalized N-aryl pyrrolidines”
Org. Biomol. Chem., 2015, 13, 2640-2651
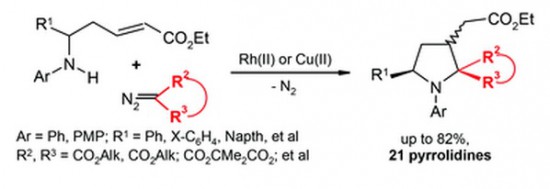
Catalytic decomposition of diazomalonates and other diazoesters using Rh(II)- and Cu(II)-complexes in the presence of α,β-unsaturated δ-(N-aryl)amino esters gives rise to the formation of multi-functionalized pyrrolidines with yields of up to 82%. The reaction apparently occurs as a domino process involving the initial N-ylide formation followed by intramolecular Michael addition to the conjugated system of amino esters to afford the pyrrolidine heterocycle. The whole process can also be classified as a [4 + 1]-annulation of the δ-amino α,β-unsaturated ester with the carbenoid intermediate.