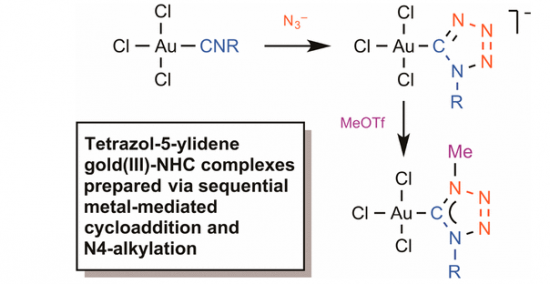
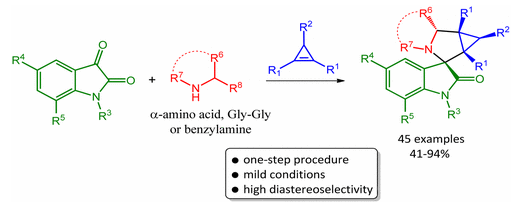
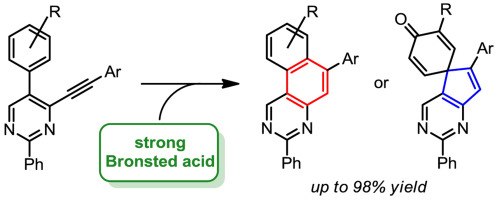
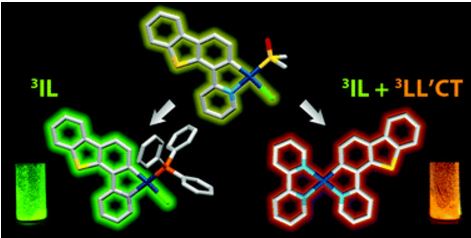
A. I. Solomatina, P.S. Chelushkin, D.V. Krupenya, I.S. Podkorytov∥, T.O. Artamonova, V.V. Sizov, A.S. Melnikov, V.V. Gurzhiy, E.I. Koshel, V.I. Shcheslavskiy, S.P. Tunik
“Coordination to Imidazole Ring Switches on Phosphorescence of Platinum Cyclometalated Complexes: The Route to Selective Labeling of Peptides and Proteins via Histidine Residues”
Bioconjugate Chem., 2017, 28 (2), 426–437.
DOI: 10.1021/acs.bioconjchem.6b00598
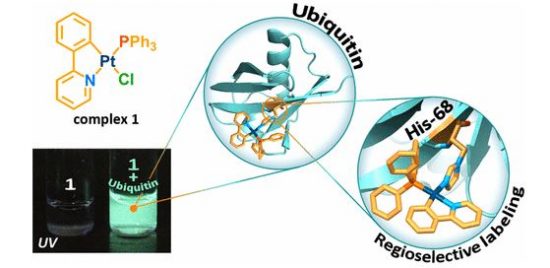
In this study, we have shown that substitution of chloride ligand for imidazole (Im) ring in the cyclometalated platinum complex Pt(phpy)(PPh3)Cl (1; phpy, 2-phenylpyridine; PPh3, triphenylphosphine), which is nonemissive in solution, switches on phosphorescence of the resulting compound. Crystallographic and nuclear magnetic resonance (NMR) spectroscopic studies of the substitution product showed that the luminescence ignition is a result of Im coordination to give the [Pt(phpy)(Im)(PPh3)]Cl complex. The other imidazole-containing biomolecules, such as histidine and histidine-containing peptides and proteins, also trigger luminescence of the substitution products. The complex 1 proved to be highly selective toward the imidazole ring coordination that allows site-specific labeling of peptides and proteins with 1 using the route, which is orthogonal to the common bioconjugation schemes via lysine, aspartic and glutamic acids, or cysteine and does not require any preliminary modification of a biomolecule. The utility of this approach was demonstrated on (i) site-specific modification of the ubiquitin, a small protein that contains only one His residue in its sequence, and (ii) preparation of nonaggregated HSA-based Pt phosphorescent probe. The latter particles easily internalize into the live HeLa cells and display a high potential for live-cell phosphorescence lifetime imaging (PLIM) as well as for advanced correlation PLIM and FLIM experiments.