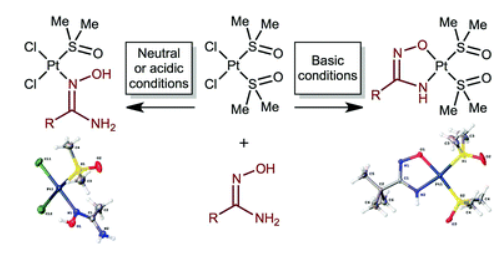
D.S. Bolotin, M.Ya. Demakova, A.A. Legin, V.V. Suslonov, A.A. Nazarov, M.A. Jakupec, B.K. Kepplerb, V.Yu. Kukushkin
“Amidoxime platinum(II) complexes: pH-dependent highly selective generation and cytotoxic activity”
NewJ.Chem., 2017, 41, 6840
DOI: 10.1039/c7nj00982h
The reaction of cis-[PtCl2(Me2[S with combining low line]O)2] with 1 equiv. of each of the amidoximes RC(NH2)[double bond, length as m-dash]NOH in neutral media in MeOH results in the formation of complexes cis-[PtCl2{RC(NH2)[double bond, length as m-dash][N with combining low line]OH}(Me2[S with combining low line]O)] (5 examples; 83–98% isolated yields). In the presence of 2 equiv. of NaOH in MeOH solution, the reaction of cis-[PtCl2(Me2[S with combining low line]O)2] with 1 equiv. of each of the amidoximes RC(NH2)[double bond, length as m-dash]NOH leads to [Pt{RC([N with combining low line]H)[double bond, length as m-dash]N[O with combining low line]}(Me2[S with combining low line]O)2] (7 examples; 74–95% isolated yields). All new complexes were characterized by C, H, and N elemental analyses, HRESI+-MS, IR, 1H, 13C{1H}, and CP-MAS TOSS 13C{1H} NMR spectroscopies, and additionally by single-crystal XRD (for seven species). The cytotoxic potency of six compounds was determined in the human cancer cell lines CH1/PA-1, A549, SK-BR-3, and SW480. Generally, the second class of complexes containing chelating amidoximato ligands shows much higher cytotoxicity than the non-chelate amidoxime analogs, despite the lack of easily exchangeable chlorido ligands. Especially, the complex [Pt(p-CF3C6H4C([N with combining low line]H)[double bond, length as m-dash]N[O with combining low line])(Me2[S with combining low line]O)2] displays a remarkable activity in the inherently cisplatin resistant SW480 cell line (0.51 μM vs. 3.3 μM).