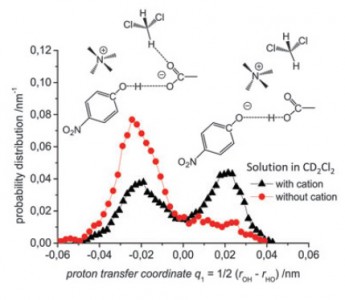
M.S. Novikov, A.F. Khlebnikov, N.V. Rostovskii, S.Tcyrulnikov, A.A. Suhanova, K.V. Zavyalov, D.S. Yufit
“Pseudopericyclic 1,5- versus Pericyclic 1,4- and 1,6-Electrocyclization in Electron-Poor 4‑Aryl-2-azabuta-1,3-dienes: Indole Synthesis from 2H‑Azirines and Diazo Compounds”
J Org. Chem., 2015, 80, 18-29
DOI: 10.1021/jo501051n
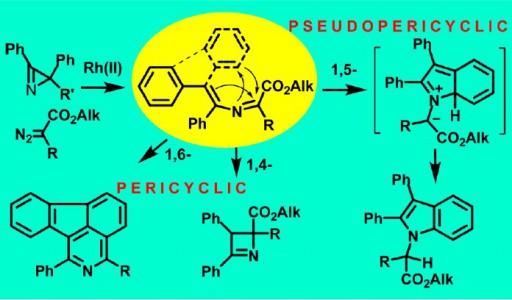
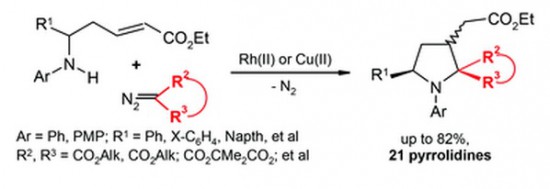
Transformations of 2-azabuta-1,3-dienes, formed in Rh2(OAc)4-catalyzed reactions of diazo carbonyl compounds with 2H-azirines, dramatically depend on the nature of substituents. 4,4-Diphenyl-2-azabuta-1,3-dienes with two electron- acceptor substituents at C1 undergo thermal 1,5-cyclization to give indoles in good yields.
The increase in electronwithdrawing ability of C1-substituents facilitates the reaction that proceeds via pseudopericyclic 1,5-electrocyclization of 2- azabutadiene into 7aH-indolium ylide followed by prototropic shift. 3,4-Diphenyl-2-azabuta-1,3-dienes, resulting from reaction of 2,3-diphenyl-2H-azirine and diazo compounds, do not produce indoles via 1,5-cyclization due to the trans-configuration of the 4-Ph-group and the nitrogen, but undergo 1,4-cyclization to 2,3- dihydroazetes. 1,6-Cyclization into 2H-1,4-oxazines with participation of the oxygen of ester or amide group at C1 of corresponding 2-azabuta-1,3-dienes does not take place due to kinetic and thermodynamic reasons. Instead of this, 1,6-electrocyclization with participation of phenyl substituent at C4 of the 2-azabuta-1,3- dienes, providing isoquinoline derivatives, can occur at elevated temperatures. The DFT-calculations (mPWB1K/6-31+G(d,p)) confirm the dependence of 2-azabuta-1,3-diene transformation type on the nature of substituents.