Center’s guest

Guest of the Center Prof. Dr. Mark Sigalov from Ben-Gurion University of the Negev (Beer Sheva, Israel) is working on the joint publication.
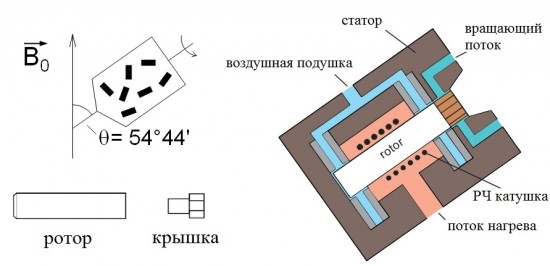
Brief overview of basic Solid-State NMR experiments
Tetrahedron Lett. 2015, 56, 5714-5717
D.I. Nilov, A.V. Vasilyev
“One-pot tandem hydrophenylation and ionic hydrogenation of 3-phenylpropynoic acid derivatives under superelectrophilic activation”
Tetrahedron Lett., 2015, 56, 5714-5717
DOI:10.1016/j.tetlet.2015.09.026
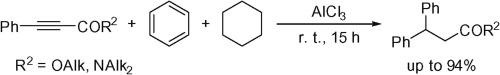
The reactions of esters and amides of 3-phenylpropynoic acid with strong Lewis acids AlX3 (X = Cl, Br) or conjugate Brønsted–Lewis superacids HX-AlX3 (X = Cl, Br) in benzene and cyclohexane at room temperature afforded 3,3-diphenylpropanoic acid derivatives in up to 94% yield. This tandem reaction of the acetylene bond proceeded by hydrophenylation followed by ionic hydrogenation.
J Org. Chem. 2015, 80, 9506-9517
A.N. Kazakova, R.O. Iakovenko, I.A. Boyarskaya, V.G. Nenajdenko, A.V. Vasilyev
“Acid-Promoted Reaction of Trifluoromethylated Allyl Alcohols with Arenes. Stereoselective Synthesis of CF3‑Alkenes and CF3‑Indanes”
J Org. Chem., 2015, 80, 9506-9517
DOI:10.1021/acs.joc.5b01398
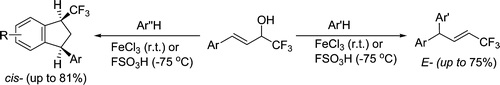
Reaction of 4-aryl-1,1,1-trifluorobut-3-en-2-ols [CF3-allyl alcohols, ArCH═CHCH(OH)CF3] with arenes under activation with anhydrous FeCl3 or FSO3H was studied. We found that the transformation led to trifluoromethylated alkenes [Ar(Ar′)CHCH═CHCF3] or 1-trifluoromethylated indanes (CF3-indanes). The formation of these two types of reaction products strongly depends on the nucleophilicity of the starting arene and the electrophilicity of cationic intermediates generated from CF3-allyl alcohols under reaction conditions. Benzene, anisole, veratrole, and ortho-xylene lead exclusively to CF3-alkenes with an E-configuration. More π-donating polymethylated arenes (pseudocumene, mesitylene) afford only CF3-indanes with a predominantly cis-orientation of substituents at positions 1 and 3 of the indane ring. Meta- and para-xylenes show an intermediate behavior; they may form both CF3-alkenes and/or CF3-indanes. The mechanisms of the investigated transformations are discussed.
September
Total in September 716 service applications were carried out.
All together measured:
- 1237 1H spectra
- 204 13C spectra
- 87 DEPT spectra
- 10 COSY spectra
- 17 NOESY spectra
- 70 31P spectra
- 99 19F spectra
128 applications were carried out.
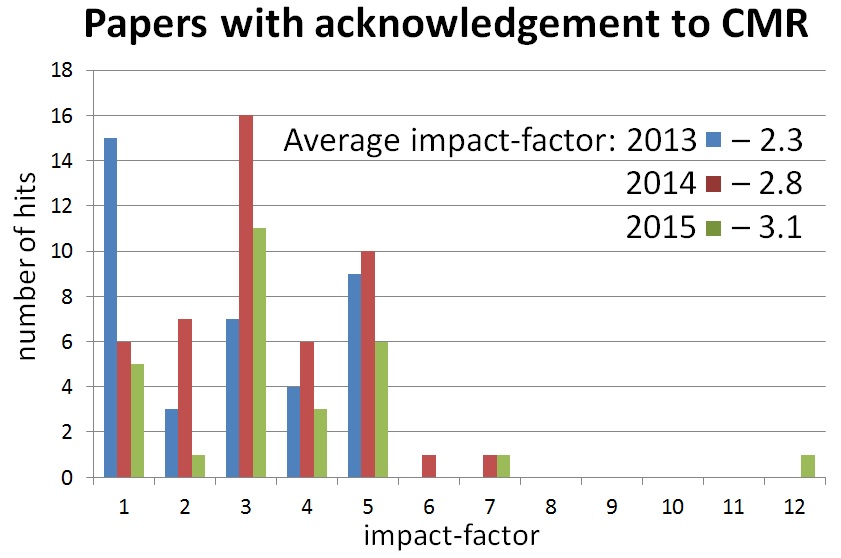
Statistics of users publications
Seminar, Ivan Giba and Katharine Grebenyuk

New employees of Center for Magnetic Resonance Ivan Giba and Katharine Grebenyuk have briefly presented they research iterests.
Guest from Puebla

Carlos Ernesto Avila Crisostomo from Institute for Physics “Ing. Luis Rivera Terrazas” (Puebla, Mexico) investigates magnetic properties of nanostructures based on synthetic opals.
Guests from INEOS RUS

Gleb A. Silantyev from the Laboratory for Metal Hydrides (A.N. Nesmeyanov Institute of Organoelement Compounds Russian Academy of Sciences) investigates dihydrogen bonds of transition metal hydrides.