New Year samples

Thanks to our users for creating a New Year’s entourage!
Tetrahedron, 2015 (71) 1940-1951
A.V. Galenko, A.F. Khlebnikov, M.S. Novikov, M.S. Avdontseva
“Synthesis of 3-(1,2-dioxoethyl)- and 2,3-dicarbonyl-containing pyrroles”
Tetrahedron, 2015, 71, 1940-1951
DOI:10.1016/j.tet.2015.02.030
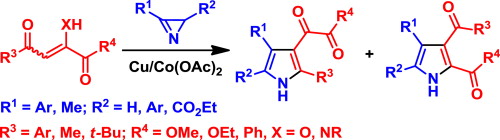
The transition metal-catalyzed reaction of 2H-azirines with 1,2,4-tricarbonyl compounds leads to 3-(1,2-dioxoethyl)- and 2,3-dicarbonyl-pyrrole derivatives, useful building blocks for the preparation of 3-heterocyclyl pyrroles and pyrroles fused with heterocycles. The influence of catalysts and the reaction conditions on the yields of pyrroles and the regioselectivity of the reaction were studied. Experimental and theoretical results suggest that the reaction proceeds via the formation of an intermediate azirine–metal complex and subsequent nucleophilic N–C3 bond cleavage.
Excursion for first year students

Mikhail Vovk has conducted a lecture for first year students of physical faculty.
Guests from Zhengzhou (China)

Zhou To and his colleagues (Centre of Education of the Russian language, Zhengzhou University) have visited Center for Magnetic Resonance.
Berlin, workshop
Peter Tolstoy has presented a lecture “SPbU Research Park, Center for Magnetic Resonance: possibilities of joint research projects” at the Berlin – St. Petersburg Workshop on Structure and Dynamics of Nanoscopic Matter.
J. Org. Chem. 2015, 80, 5546-5555
N.A. Danilkina, A.G. Lyapunova, A.F. Khlebnikov, G.L. Starova, S. Brase, I.A. Balova
“Ring-Closing Metathesis of Co2(CO)6–Alkyne Complexes for the Synthesis of 11-Membered Dienediynes: Overcoming Thermodynamic Barriers”
J. Org. Chem., 2015, 80, 5546-5555
DOI:10.1021/acs.joc.5b00409
The feasibility of ring-closing metathesis (RCM) as a synthetic entry to 10- and 11-membered dienediynes fused to a benzothiophene core was explored by experimental and theoretical investigations. An established sequence of iodocyclization of o-(buta-1,3-diynyl)thioanisoles followed by Sonogashira coupling to form diethynylbenzothiophenes was used to synthesize terminal benzothiophene-fused enediyne diolefins as substrates for RCM. Encountering an unexpected lack of reactivity of these substrates under standard RCM conditions, we applied DFT calculations to reveal that the underlying cause was a positive change in Gibbs free energy. The change in Gibbs free energy was also found to be positive for RCM of indole- and benzannulated terminal diolefins when affording smaller than 12-membered rings. We found that modification of the enediyne–diolefin substrate as the Co2(CO)6–alkyne complex allowed the target benzothiophene-fused 11-membered dienediyne to be obtained via RCM; the alkyne complexation strategy therefore provides one valid technique for overcoming challenges to macrocyclization of this kind.
University of Jyväskylä
RSC Advances, 2015
O.A. Tomashenko, A.F. Khlebnikov, I.P Mosiagin, M.S. Novikov, E.V. Grachova, J.R. Shakirova, S.P. Tunik
“A new heterocyclic skeleton with highly tunable absorbtion/emission wavelength via H-bonding”
RSC Adv., 2015, accepted
DOI:10.1039/C5RA17755C
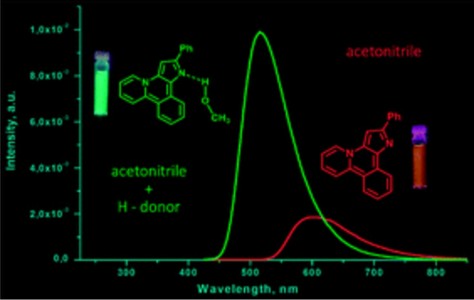
A new heterocyclic system, pyrido[2,1-a]pyrrolo[3,2-c]isoquinoline, was synthesized via Pd-catalyzed intramolecular cyclization of 1-[1-benzyl-2-(2-bromophenyl)-1H-pyrrol-3-yl]pyridin-1-ium bromides. The heterocycles obtained display stimuli responsive fluorescence in solution depending on the nature of the solvent. The strongest blue shift of the emission maxima and growth in luminescence intensity was observed in protic solvents and upon addition of proton donors to solutions of compounds in aprotic solvents. The effect of proton donors on emission characteristics was explained by DFT calculations in terms of H-complex formation with the nucleophilic centres of the molecular skeleton
Museum exhibit in CMR

Varian 200/51 NMR spectrometer was placed in the hall of Center for Magnetic Resonance. You will be able to look inside the spectrometer magnet system and make sure that there are no more special secrets behind the walls of the dewar.