M.Ya. Demakova, D.S. Bolotin, N.A. Bokach, R.M. Islamova, G.L. Starova, V.Yu. Kukushkin
“Click-Type PtII-Mediated Hydroxyguanidine–Nitrile Coupling Provides Useful Catalysts for Hydrosilylation Cross-Linking”
ChemPlusChem, 2015, 80, 1607-1614
DOI:10.1002/cplu.201500327
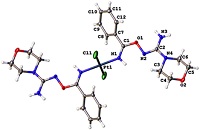
The nitrile complexes trans-[PtCl2(RCN)2] (R=Et (NC1), tBu (NC2), Ph (NC3), p-BrC6H4 (NC4)) and cis-[PtCl2(RCN)2] (R=Et (NC5), tBu (NC6), Ph (NC7)) react with 1 equiv of the hydroxyguanidine OC4H8NC(=NOH)NH2 (HG) furnishing the mono-addition products trans- and cis-[PtCl2(RCN){NH=C(R)ON=C(NH2)NC4H8O}] (1–4 and 9–11; 7 examples; 54–74 % yield). Treatment of any of the nitrile complexes NC1–NC7 with HG in a 1:2 molar ratio generated the bis-addition products trans- and cis-[PtCl2{NH=C(R)ON=C(NH2)NC4H8O}2] (5–8 and 12–14; 7 examples; 69–89 % yield). The PtII-mediated coupling between nitrile ligands and HG proceeds substantially faster than the corresponding reactions involving amid- and ketoximes and gives redox stable products under normal conditions. Complexes 1, 6⋅4 CH2Cl2, 7⋅4 CH2Cl2, 8⋅2 CH2Cl2, and NC4 were studied by X-ray crystallography. Platinum(II) species 1–3, 10, 11, and especially 9, efficiently catalyze the hydrosilylation cross-linking of vinyl-terminated poly(dimethylsiloxane) and trimethylsilyl-terminated poly(dimethylsiloxane-co-ethylhydrosiloxane) giving high-quality thermally stable silicon resins with no structural defects. The usage of these platinum species as the catalysts does not require any inhibitors and, moreover, the complexes and their mixtures with vinyl- and trimethylsilyl-terminated polysiloxanes are shelf-stable in air.