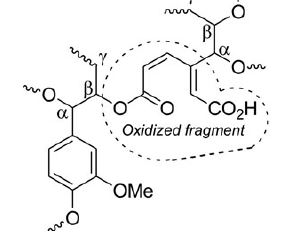
A.Ya. Bespalov, T.L. Gorchakova, A.Yu. Ivanov, M.A. Kuznetsov, L.M. Kuznetsova, A.S. Pan’kova, L.I. Prokopenko, A.F. Khlebnikov
“On the Possibility for Synthesizing Dihydrotriazolothiadiazoles by Condensation of 4-Amino-2,4-dihydro-3H-1,2,4-triazole-3-thiones with Aromatic Aldehydes”
Russ J Org Chem., 2016, 52, 421-428
DOI:10.1134/S1070428016030210
Regardless of the conditions, the condensation of 4-amino-2,4-dihydro-3H-1,2,4-triazole-3-thiones with aromatic aldehydes afforded the corresponding hydrazones as the only product. Both initial amines and resulting hydrazones exist as the thione rather than thiol tautomer. In no case bicyclic 5,6-dihydro[1,2,4]triazolo[3,4-b][1,3,4]thiadiazoles that are isomeric to the hydrazones were detected. DFT quantum chemical calculations at the B3LYP/6-31+g(d,p) level of theory with full geometry optimization showed that the hydrazone structure in methanol and DMF is more stable than the bicyclic isomer by 19–23 kcal/mol, which completely excluded the possibility for such cyclization. The thione tautomer of the hydrazones is more stable than the thiol structure by 11–13 kcal/mol.