D.M. Ivanov, P.V. Gushchin, A.S. Novikov, M.S. Avdontceva, A.A. Zolotarev, G.L. Starova, Y.-T. Chen, S.-H. Liu, P.-T. Chou, V.Yu. Kukushkin
“Platinum(II)-Mediated Double Coupling of 2,3-Diphenylmaleimidine with Nitrile Functionalities To Give Annulated Pentaazanonatetraenate (PANT) Systems”
Eur. J. Inorg. Chem., 2016, 1480–1487
DOI:10.1002/ejic.201501398

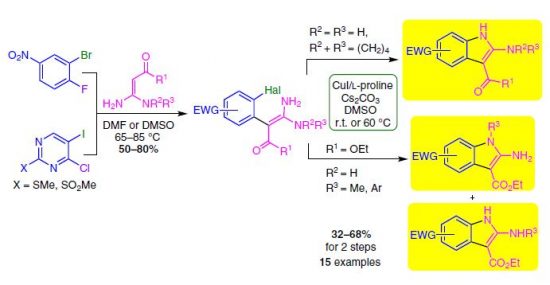
Treatment of trans-[PtCl2(NCR)2] [R = Et 1, nPr 2, tBu 3, CH2Ph 4, Ph 5, p-CF3C6H4 6, NMe2 7, NEt2 8, N(CH2)5 9] with 2.5 equiv. of 2,3-diphenylmaleimidine in CH2Cl2 at room temperature for 5 min [for R = p-CF3C6H4 6, NMe2 7, NEt2 8, N(CH2)5 9] or 14 h (for R = Et 1, nPr 2, tBu 3, CH2Ph 4, Ph 5) furnishes (1,3,5,7,9-pentaazanona-1,3,6,8-tetraenato) platinum(II) [(PANT)PtII; PtCl{HN=C(R)N=CN[C(Ph)=C(Ph)]C=NC(R)=NH}] complexes 10–18. These species are formed by platinum(II)-mediated double coupling of 2,3-diphenylmaleimidine with both nitrile ligands. The formulation of the complexes was supported by satisfactory C, H, and N elemental analyses, which were in agreement with HRESI-MS, IR, and 1H and 13C{1H} NMR spectra. The structures of 10, 11·1/8nC6H14, 15, 16·CCl4, and 17·CHCl3 were determined by single-crystal X-ray diffraction. Absorption and emission studies were performed on representative complexes 10, 15, and 18, and the results show weak phosphorescence maxima at around 730–750 and 770–820 nm in CH2Cl2 and in the solid state, respectively. Further insight into the photophysical properties was gained by time-dependent density functional theory (TD–DFT) with detailed analysis of the corresponding frontier molecular orbitals for the lower-lying transition. The calculated energies of the T1 state (in terms of wavelength) are 715.8 nm for 10, 764.6 nm for 15, and 697.7 nm for 18, and these values are in good agreement with the trend of the first vibronic peaks of their phosphorescence spectra.