Stanislav Sukharzhevskii and Mikhail Vovk have presented a lecture-excursion for second year students of the Faculty of Physics.
Excursion
Workshop in cmr
Our new colleagues have presented short talks about their areas of interest and scientific experience from previous education/work places:
- Roman Belykh “In-situ Analysis of nickel catalysts during CO2 methanation with FT-IR spectroscopy”
- Demidov Evgeniy “Surface active monomers application for carbon nanotubes – polymers composites preparation”
- Irina Alisova “Investigation suppression and generation mechanisms of triplet states for organic molecules”
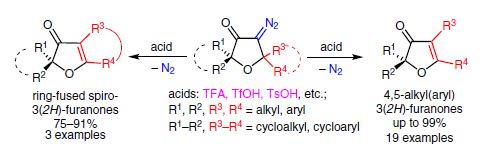
Synthesis, 2016, 48, A-H
J.J. Medvedev, D.V. Semenok, X.V. Azarova, L.L. Rodina, V.A. Nikolaev
“A New Powerful Approach to Multi-Substituted 3(2H)-Furanones via Brønsted Acid-Catalyzed Reactions of 4-Diazodihydrofuran-3-ones”
Synthesis, 2016, 48, A-H
DOI:10.1055/s-0036-1588304
The interaction of 5,5-dialkyl(diaryl)-substituted 4-diazo-3(2H)furanones with Brønsted acids (TFA, TsOH, etc.) causes elimination of nitrogen accompanied by 1,2-nucleophilic rearrangement, giving rise to exclusive formation of 4,5-dialkyl(diaryl)-substituted 3(2Н)-furanones, ring-fused 3(2H)-furanones, and phenanthro[9,10-b]furan-3(2H)-ones in yields of up to 99%. The reaction is a new highly efficient way for the synthesis of multisubstituted 3(2Н)furanones.
Training course
Helvetica Chim. Acta, 2016, 99, 716-723
D.V. Semenok, J.J. Medvedev, M.S. Avdontceva, S.I. Selivanov, J. Sieler, A.S. Mereshchenko, V.A. Nikolaev
“Experimental Evidence of Intramolecular CAr–H···O=C Hydrogen Bonds in the Structure of (Diaryl)tetrahydrofuranones Using Spectroscopic Tools”
Helvetica Chim. Acta, 2016, 99, 716-723
DOI:10.1002/hlca.201600149
The occurrence of bifurcate H-bonds CAr–H···O=C in the structure of (diaryl)-tetrahydrofuranones was experimentally demonstrated using different methods and techniques. The consistent increasing spin–spin coupling constants 1J(C,H) of the ortho-H-atoms and low-field shift of v (C=O) in IR spectra of 2,2-(diaryl)tetrahydrofuran-3(2H)-ones relative to their 5,5-diaryl counterparts, as well as pronounced dependence of the ortho-C–H H-atoms chemical shifts on the temperature and solvent polarity along with X-ray diffraction analysis data unambiguously point to the existence of weak CAr–H···O=C H-bonds in these molecules.
Bioconjugate Chem, 2016, 27, 143-150
A.A. Belyaev, D.V. Krupenya, E.V. Grachova, V.V. Gurzhiy, A.S. Melnikov, P.Yu. Serdobintsev, E.S. Sinitsyna, E.G. Vlakh, T.B. Tennikova, S.P. Tunik
“Supramolecular AuI–CuI Complexes as New Luminescent Labels for Covalent Bioconjugation”
Bioconjugate Chem, 2016, 27, 143-150
DOI:10.1021/acs.bioconjchem.5b00563
Two new supramolecular organometallic complexes, namely, [Au6Cu2(C2C6H4CHO)6(PPh2C6H4PPh2)3](PF6)2 and [Au6Cu2(C2C6H4NCS)6(PPh2C6H4PPh2)3](PF6)2, with highly reactive aldehyde and isothiocyanate groups have been synthesized and characterized using X-ray crystallography, ESI mass spectrometry, and NMR spectroscopy. The compounds obtained demonstrated bright emission in solution with the excited-state lifetime in microsecond domain both under single- and two-photon excitation. The luminescent complexes were found to be suitable for bioconjugation in aqueous media. In particular, they are able to form the covalent conjugates with proteins of different molecular size (soybean trypsin inhibitor, human serum albumin, rabbit anti-HSA antibodies). The conjugates demonstrated a high level of the phosphorescent emission from the covalently bound label, excellent solubility, and high stability in physiological media. The highest quantum yield, storage stability, and luminance were detected for bioconjugates formed by covalent attachment of the aldehyde-bearing supramolecular AuI–CuI complex. The measured biological activity of one of the labeled model proteins clearly showed that introduced label did not prevent the biorecognition and specific protein–protein complex formation that was extremely important for the application of the conjugates in biomolecular detection and imaging.
Inorg. Chem., 2016, 55, 4720-4732
Статья пользователей РЦ МРМИ, попавшая на обложку журнала Inorganic Chemistry:
A.A. Penney, V.V. Sizov, E.V. Grachova, D.V. Krupenya, V.V. Gurzhiy, G.L. Starova, S.P. Tunik
“Aurophilicity in Action: Fine-Tuning the Gold(I)–Gold(I) Distance in the Excited State To Modulate the Emission in a Series of Dinuclear Homoleptic Gold(I)–NHC Complexes”
Inorg. Chem., 2016, 55, 4720-4732
DOI:10.1021/acs.inorgchem.5b02722
The formation of exciplexes between cationic dinuclear Au(I) bis(carbene) complexes and bromide ions was found to decrease the Au−Au distance in the triplet excited state, leading to pronounced changes in emission. The stimuli-responsive nature of the intracationic aurophilic interaction paves the way for fine-tuning the emission energy and opens new possibilities for practical applications.
Trophy of training course

Michael Vovk has successfully completed the training course “Advanced NMR Methods”. The course covered the uses of gradients, different options of shimming, set of 1D, 2D and 3D homonuclear and heteronuclear correlation experiments with gradient selection and water suppression, other aspects of modern NMR methods.
Congress of the University of Arctic
Within the framework of the Congress of the University of Arctic (12-16.09.2016, St.Petersburg; http://www.uarctic.org/about-uarctic/events/uarctic-congress-2016/) Peter Tolstoy has given a presentation about the Research Park on the example of the Center for Magnetic Resonance. During the presentation a video-conference has been established, where CMR specialists Anton Mazur and Mikhail Vovk answered the questions about the current projects and the overall workload.