Dr. Konstantinos Sotiriadis (Institute of Theoretical and Applied Mechanics, Academy of Sciences of the Czech Republic) has presented a short lecture about his scientific work and current investigations in CMR.
Workshop in CMR
Spinus-2016

An Excursion for participants of 13 International Youth School-Conference “Magnetic resonance and its applications – Spinus-2016” was conducted in CMR. A modern NMR, EPR, NQR equpments and some current investigations were demonstrated for our visitors. Peter Tolstoy has presented to the participants of Spinus-2016 a lecture titled “Cooperativity of Strong Hydrogen Bonds Studied by Liquid State NMR Spectroscopy”
J. Org. Chem., 2016, 81, 11210-11221
L.D. Funt, O.A. Tomashenko, A.F. Khlebnikov M.S. Novikov, A.Yu. Ivanov
“Synthesis, Transformations of Pyrrole- and 1,2,4-Triazole-Containing Ensembles, and Generation of Pyrrole-Substituted Triazole NHC”
J. Org. Chem., 2016, 81, 11210-11221
DOI:10.1021/acs.joc.6b02200
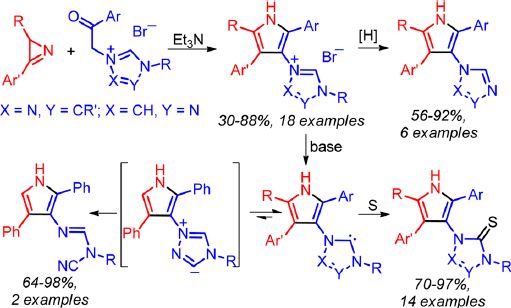
Unprecedented pyrrole- and 1,2,4-triazole-containing ensembles, substituted 1-(1H-pyrrol-3-yl)-4H-1,2,4-triazol-1- ium bromides and 4-(1H-pyrrol-3-yl)-1H-1,2,4-triazol-4-ium bromides, were prepared from 2H-azirines and triazolium phenacyl bromides using a simple procedure. N-(1H-Pyrrol-3-yl)-N′-benzyltriazolium bromides undergo reductive debenzylation on Pd/C to give substituted 1-(1H-pyrrol-3-yl)-4H-1,2,4-triazoles and 4-(1H-pyrrol-3-yl)-1H-1,2,4-triazoles in high yields. Betaines (triazoliumylpyrrolides) and pyrrolyltriazole NHCs, which are possible products of dehydrobromination of pyrrolyltriazolium salts, have comparable thermodynamic stabilities in nonpolar solvents according to calculations at the DFT B3LYP/6-31G(d) level. The carbene forms can be easily trapped by the reaction of salts with base in the presence of sulfur. The corresponding 1- and 4-(1H-pyrrol-3-yl)-1H-1,2,4-triazole-5(4H)-thiones are formed in high yields. In the absence of sulfur as a trap, the opening of the triazole ring occurs with the formation of derivatives of N-cyanoformimidamide. According to the DFT calculations the latter is most probably formed via a pyrrolyltriazoliumide intermediate, which is the minor component of the equilibrium triazoliumylpyrrolide−pyrrolyltriazole NHC−pyrrolyltriazoliumide. Blocking of the pyrrolyltriazoliumide intermediate formation, by introduction of a substituent at the 3-position of the triazole ring, made it possible to generate the first pyrrole-substituted triazole NHC.
Eur. J. Inorg. Chem., 2016, 28, 4659-4667
E.A. Popova, T.V. Serebryanskaya, S.I. Selivanov, M. Haukka, T.L. Panikorovsky, V.V. Gurzhiy, I. Ott, R.E. Trifonov, V.Yu. Kukushkin
“Water soluble platinum(II) complexes featuring 2-alkyl-2H-tetrazol-5-ylacetic acids: synthetic, X-ray diffraction, and solution NMR studies”
Eur. J. Inorg. Chem., 2016, 28, 4659-4667
DOI:10.1002/ejic.201600626

2-R-2H-Tetrazol-5-ylacetic acids (abbreviated as 2-R-taa; R = Me, iPr, tBu) react with K2[PtCl4] in 1 m HCl in H2O at r.t. furnishing trans-platinum(II) complexes trans-[PtCl2(2-R-taa)2] (1–3), whereas cis-isomeric species cis-[PtCl2(2-R-taa)2] (R = iPr, 4; tBu, 5) are isolated at lower temperature (4–6 °C). In the presence of EtOH in the reaction mixture, esterification of the tetrazol-5-ylacetoxy group of 2-tBu-taa leads to trans-[PtCl2(ethyl 2-tert-butyl-2H-tetrazol-5-ylacetate)2] (6). Complexes 1–6 were characterized by elemental analyses (CHN), HRESI+-MS, 1H, 13C{1H}, 195Pt{1H} NMR and IR spectroscopy, differential scanning calorimetry/thermogravimetry (DSC/TG), and X-ray diffraction (for 1·H2O, 2, 3·2H2O, 4, 5·2H2O, and 6). The generation of the tetrazole-based complexes in solution (1 m DCl in D2O, 25 °C) was studied by 1H NMR spectroscopy and HPLC-MS. The obtained data indicate the initial formation of anionic [PtCl3(2-R-taa)]– complexes that are subsequently converted into disubstituted isomeric platinum(II) species cis- and trans-[PtCl2(2-R-taa)2]. By contrast to cis- and trans-[PtCl2(2-R-taa)2] that were inactive in two human cancer models in vitro (IC50 > 100 µm), complex 6 demonstrated noticeable antiproliferative effects in HT-29 colon and MCF-7 breast carcinoma cell lines with IC50 values of 14.2 ± 1.1 and 5.8 ± 1.2 µm, respectively.
J. Am. Chem. Soc., 2016, 138, 14129-14137
A.S. Mikherdov, M.A. Kinzhalov, A.S. Novikov, V.P. Boyarskiy, I.A. Boyarskaya, D.V. Dar’in, G.L. Starova, V.Yu. Kukushkin
“Difference in energy between two distinct types of chalcogen bonds drives regioisomerization of binuclear (Diaminocarbene)PdII complexes”
J. Am. Chem. Soc., 2016, 138, 14129-14137
DOI:10.1021/jacs.6b09133
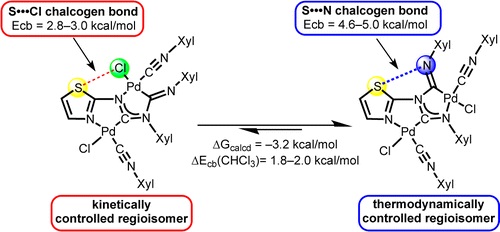
The reaction of cis-[PdCl2(CNXyl)2] (Xyl = 2,6-Me2C6H3) with various 1,3-thiazol- and 1,3,4-thiadiazol-2-amines in chloroform gives a mixture of two regioisomeric binuclear diaminocarbene complexes. For 1,3-thiazol-2-amines the isomeric ratio depends on the reaction conditions and kinetically (KRs) or thermodynamically (TRs) controlled regioisomers were obtained at room temperature and on heating, respectively. In CHCl3 solutions, the isomers are subject to reversible isomerization accompanied by the cleavage of Pd–N and C–N bonds in the carbene fragment XylNCN(R)Xyl. Results of DFT calculations followed by the topological analysis of the electron density distribution within the formalism of Bader’s theory (AIM method) reveal that in CHCl3 solution the relative stability of the regioisomers (ΔGexp = 1.2 kcal/mol; ΔGcalcd = 3.2 kcal/mol) is determined by the energy difference between two types of the intramolecular chalcogen bonds, viz. S···Cl in KRs (2.8–3.0 kcal/mol) and S···N in TRs (4.6–5.3 kcal/mol). In the case of the 1,3,4-thiadiazol-2-amines, the regioisomers are formed in approximately equal amounts and, accordingly, the energy difference between these species is only 0.1 kcal/mol in terms of ΔGexp (ΔGcalcd = 2.1 kcal/mol). The regioisomers were characterized by elemental analyses (C, H, N), HRESI+-MS and FTIR, 1D (1H, 13C{1H}) and 2D (1H,1H-COSY, 1H,1H-NOESY, 1H,13C-HSQC, 1H,13C-HMBC) NMR spectroscopies, and structures of six complexes (three KRs and three TRs) were elucidated by single-crystal X-ray diffraction.
Guests in CMR

Dr. Konstantinos Sotiriadis Institute of Theoretical and Applied Mechanics, Academy of Sciences of the Czech Republic. The work of Dr. Sotiriadis at CMR will be dedicated to the study of magnesium-containing cements by solid-state NMR spectroscopy.
Organometallics, 2016, 35, 3569-3576
T.B. Anisimova, M.A. Kinzhalov, M.L. Kuznetsov, M.F.C. Guedes da Silva, A.A. Zolotarev, V.Yu. Kukushkin, A.J.L. Pombeiro, K.V. Luzyanin
“1,3-Dipolar cycloaddition of nitrones to gold(III)-bound isocyanides”
Organometallics, 2016, 35, 3569-3576
DOI:10.1021/acs.organomet.6b00635
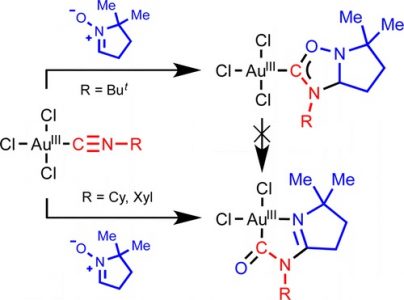
Treatment of gold(III)-isocyanides [AuCl3(CNR1)] (R1 = Xyl 1, Cy 2, But 3) with an equimolar amount of 5,5-dimethyl-1-pyrroline-N-oxide (4) in CH2Cl2 at −74 °C leads to the generation of the heterocyclic aminocarbene species [AuCl3{C(ONaCMe2CH2CH2CbH)═NeR1}(Na–Cb)(Cb–Ne)] 8 (for R1 = But) or gold(III) complexes cis-[AuCl2{Na(CMe2CH2CH2CbNeR1)Cd═O}(Na═Cb)(Ne–Cd)] 9 and 10 (for R1 = Xyl and Cy) in good isolated yields (75–87%). DFT calculations show that deprotonation of the endocyclic CH group in the carbene ligand leads to spontaneous N–O bond cleavage, and acidity of this group is a factor controlling the different chemical behavior of 1–3 depending on the nature of substituent R1. The reaction of equimolar amounts of the aldonitrone p-TolCH═N+(Me)O– (5) or the ketonitrones Ph2C═N+(R2)O– (R2 = Ph 6, CH2Ph 7) with 1–3 in CD2Cl2 at −70 °C in air (or under N2) revealed the formation of the carbene complexes [AuCl3{C(ONMeCaH-p-Tol)═NbR1}(Ca–Nb)] (R1 = Cy 11, Xyl 12, But 13), [AuCl3{C(ONPhCaPh2)═NbR1}(Ca–Nb)] (R1 = Cy 14, But 15), or [AuCl3{C(ON(CH2Ph)CaPh2)═NbR1}(Ca–Nb)] (R1 = Cy 16, Xyl 17), as studied by 1H NMR. The reaction of 6 with 1 and of 7 with 3 did not furnish carbene products. Compounds 8–10 were characterized by ESI-MS, IR, 1D (1H, 13C{H}) and 2D (1H,1H–COSY, 1H,13C-HSQC, 1H,13C-HMBC) NMR spectroscopic techniques, and, only for 8, elemental analyses (C, H, N), while compounds 11–17 were characterized by 1D (1H, 13C{H}) and 2D (1H,13C-HSQC) NMR. Structures of compounds 8, 9, and 13 were additionally established by single-crystal X-ray diffraction.
October
Total in October 2369 service applications were carried out.
All together measured:
- 2277 1H spectra
- 503 13C spectra
- 190 DEPT spectra
- 51 COSY spectra
- 17 NOESY spectra
- 99 31P spectra
- 74 19F spectra
159 applications were carried out.
Organometallics, 2016, 35, 3612-3623
D.S. Bolotin, A.S. Novikov, V.K. Burianova, M.Ya. Demakova, E.A. Daines, С. Pretorius, P. Mokolokolo, A. Roodt, N.A. Bokach, M.S. Avdontceva, A.P. Zhdanov, K.Yu. Zhizhin, N.T. Kuznetsov, V.Yu. Kukushkin
“Nucleophilicity of oximes based upon addition to а nitrilium closo-decaborate cluster”
Organometallics, 2016, 35, 3612-3623
DOI:10.1021/acs.organomet.6b00678
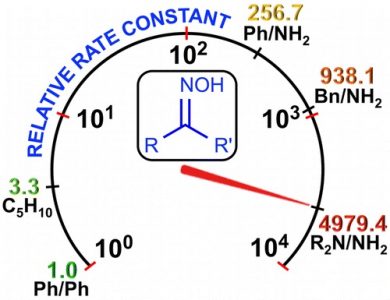
Three types of oxime species, i.e., 4-morpholylcarbamidoxime (hydroxyguanidine), phenylacetamidoxime and benzamidoxime (amidoximes), and cyclohexanone oxime and benzophenone oxime (ketoximes), react at room temperature with the 2-nitrilium closo-decaborate clusters, leading to 2-iminium closo-decaborates (14 examples; 57–94%). These species were characterized by ICPMS-based boron elemental analysis, HRESI–-MS, molar conductivity, IR, 1H{11B}, and 11B{1H} NMR spectroscopies, and additionally by single-crystal X-ray diffraction (for six compounds). On the basis of kinetic data, ΔH⧧, ΔS⧧, and ΔG⧧ of the additions were determined, showing a 4 order-of-magnitude decrease in reactivity from the hydroxyguanidine to the aromatic ketoxime as entering nucleophiles. The results of DFT calculations indicate that the mechanism for these reactions is stepwise and is realized through the formation of the orientation complex of the nitrone form, R2R3C═N+(H)O–, of oximes with [B10H9NCEt]−, giving further an acyclic intermediate (the rate-determining step), followed by proton migration, leading to the addition product. The calculated overall activation barrier for these transformations is consistent with the experimental kinetic observations. This work provides, for the first time, a broad nucleophilicity series of oximes, which is useful to control various nucleophilic additions of oxime species.