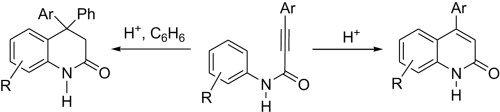
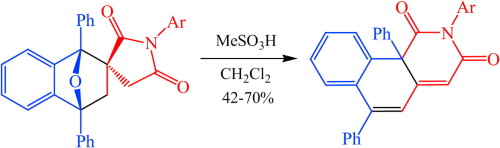
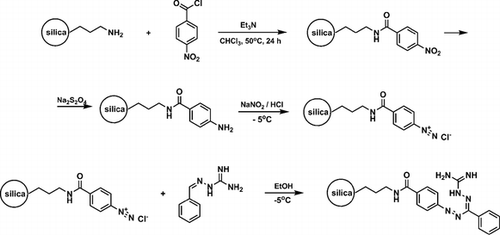
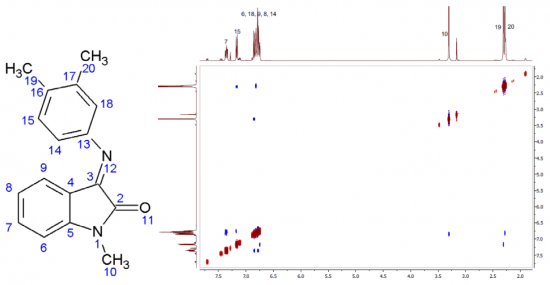
A.S. Smirnov, E.S. Butukhanova, N.A. Bokach, G.L. Starova, V.V. Gurzhiy, M.L. Kuznetsov, V.Yu. Kukushkin
“Novel (cyanamide)ZnII complexes and zinc(II)-mediated hydration of the cyanamide ligands”
Dalton Trans, 2014, 43, 15798-15811
DOI:10.1039/c4dt01812e
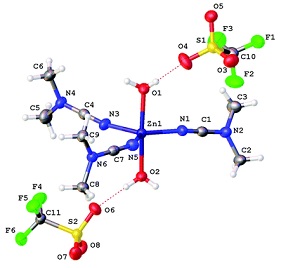
The cyanamides NCNR2 (R2 = Me2, Ph2, C5H10) react with ZnX2 (X = Cl, Br, I) in a 2[thin space (1/6-em)]:[thin space (1/6-em)]1 molar ratio at RT, giving a family of zinc(II) complexes [ZnX2(NCNR2)2] (R2 = Me2, X = Cl 1, X = Br 2, X = I 3; R2 = C5H10, X = Cl 4, X = Br 5; X = I 6; R2 = Ph2, X = Cl 7, X = Br 8, X = I 9; 75–92% yields). Complexes 7 and 8 undergo ligand redistribution in wet CH2Cl2 solutions giving the [Zn(NCNPh2)4(H2O)2][Zn2(μ-X)2X4] (X = Cl 10, Br 11) species that were characterized by 1H NMR, HRESI-MS, and X-ray diffraction. Halide abstraction from 1–3 by the action of AgCF3SO3 or treatment of Zn(CF3SO3)2 with NCNR2 (R2 = Me2, C5H10) leads to labile complexes [Zn(CF3SO3)2(NCNR2)3] (R2 = Me2, 12; C5H10, 13). Crystallization of 12 from wet CH2Cl2 or from the reaction mixture gave [Zn(NCNMe2)3(H2O)2](SO3CF3)2 (12a) or [Zn(CF3SO3)2(NCNMe2)2]∞ (12b), whose structures were determined by X-ray diffraction. The ZnII-mediated hydration was observed for the systems comprising ZnX2 (X = Cl, Br, I), 2 equiv. NCNR2 (R2 = Me2, C5H10, Ph2) and ca. 40-fold excess of water and conducted in acetone at 60 °C (R2 = Me2, C5H10) or 80 °C (R2 = Ph2) in closed vials, and it gives the urea complexes [ZnX2{OC(NR2)NH2}] (R2 = Me2, X = Cl 13, X = Br 14, X = I 15; R = C5H10, X = Cl 16, X = Br 17; X = I 18; R2 = Ph2, X = Cl 19, X = Br 20, X = I 21; 57–81%). In contrast to the ZnII-mediated hydration of conventional nitriles, which proceeds only in the presence of co-catalyzing oximes or carboxamides, the reaction with cyanamides does not require any co-catalyst. Complexes 1–9, 12–19 were characterized by 1H, 13C{1H} NMR, IR, HRESI-MS, and X-ray crystallography (for 1–3, 8, 9, 13–15, and 17), whereas 20 and 21 were characterized by HRESI+-MS and 1H and 13C{1H} NMR (for 20). The structural features of the cyanamide complexes 1, 2, 7, and 8 were interpreted by theoretical calculations at the DFT level.