E.A. Katlenok, A.A. Zolotarev, A.Yu. Ivanov, S.N. Smirnov, R.I. Baichurin, K.P. Balashev
“Complexes of Ir(III) and Pt(II) with Cyclometallated 2-Phenylbenzothiazole and Chelating Diethyldithiocarbamate and O-Ethyldithiocarbonate Ions: Structures and Optical and Electrochemical Properties”
Rus. J. Coord. Chem., 2016, 42, 178-186
DOI:10.1134/S1070328416030039
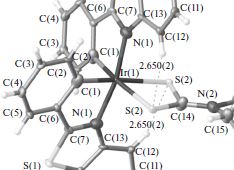
It is shown by X-ray diffraction analysis, IR spectroscopy, and 1Н, 13С{1H}, and 195Pt NMR spectroscopy that the Pt(II) and octahedral Ir(III) complexes with metallated 2-phenylbenzothiazole and chelating diethyldithiocarbamate and O-ethyldithiocarbonate ions have the square and cis-C,C structure, respectively. The highest occupied and lowest unoccupied molecular orbitals of the complexes determining their long-wavelength absorption, phosphorescence, and one-electron oxidation and reduction are assigned to those predominantly localized on the mixed p(S)/d(M) and π* orbitals of the metallated ligand. The cathodic shift of the oxidation voltammogram and the bathochromic phosphorescence shift of the Pt(II) complex with the О-ethyldithiocarbonate ion are attributed to the enhanced donor–acceptor interaction of the donor S atoms of the ligand with Pt(II). The structural data are deposited with the Cambridge Crystallographic Data Centre (CIF files CCDC nos. 1058768 (Ia) and 1058767 (IIb)).