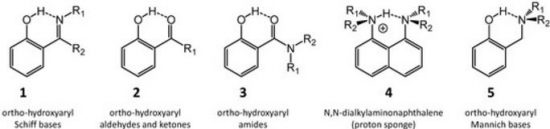
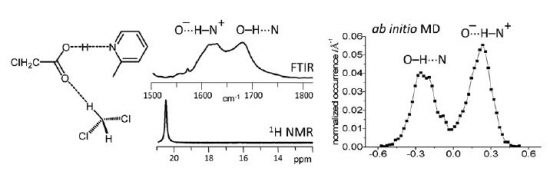
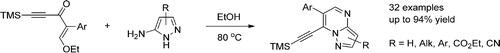
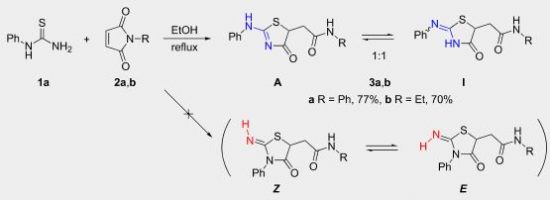
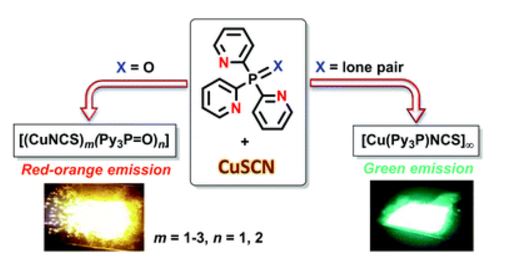
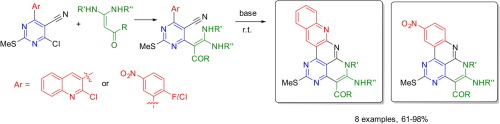
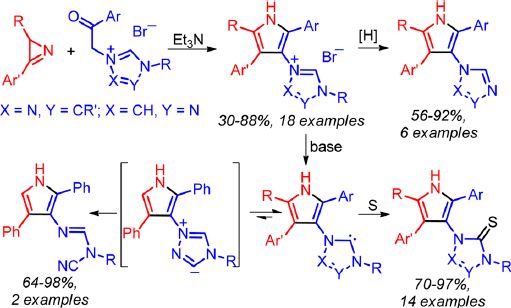
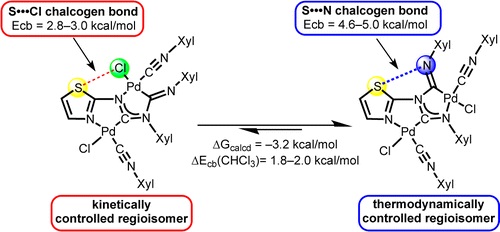
T.B. Anisimova, M.A. Kinzhalov, M.L. Kuznetsov, M.F.C. Guedes da Silva, A.A. Zolotarev, V.Yu. Kukushkin, A.J.L. Pombeiro, K.V. Luzyanin
“1,3-Dipolar cycloaddition of nitrones to gold(III)-bound isocyanides”
Organometallics, 2016, 35, 3569-3576
DOI:10.1021/acs.organomet.6b00635
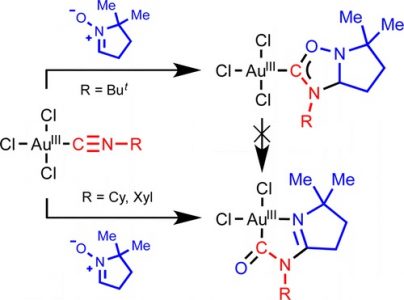
Treatment of gold(III)-isocyanides [AuCl3(CNR1)] (R1 = Xyl 1, Cy 2, But 3) with an equimolar amount of 5,5-dimethyl-1-pyrroline-N-oxide (4) in CH2Cl2 at −74 °C leads to the generation of the heterocyclic aminocarbene species [AuCl3{C(ONaCMe2CH2CH2CbH)═NeR1}(Na–Cb)(Cb–Ne)] 8 (for R1 = But) or gold(III) complexes cis-[AuCl2{Na(CMe2CH2CH2CbNeR1)Cd═O}(Na═Cb)(Ne–Cd)] 9 and 10 (for R1 = Xyl and Cy) in good isolated yields (75–87%). DFT calculations show that deprotonation of the endocyclic CH group in the carbene ligand leads to spontaneous N–O bond cleavage, and acidity of this group is a factor controlling the different chemical behavior of 1–3 depending on the nature of substituent R1. The reaction of equimolar amounts of the aldonitrone p-TolCH═N+(Me)O– (5) or the ketonitrones Ph2C═N+(R2)O– (R2 = Ph 6, CH2Ph 7) with 1–3 in CD2Cl2 at −70 °C in air (or under N2) revealed the formation of the carbene complexes [AuCl3{C(ONMeCaH-p-Tol)═NbR1}(Ca–Nb)] (R1 = Cy 11, Xyl 12, But 13), [AuCl3{C(ONPhCaPh2)═NbR1}(Ca–Nb)] (R1 = Cy 14, But 15), or [AuCl3{C(ON(CH2Ph)CaPh2)═NbR1}(Ca–Nb)] (R1 = Cy 16, Xyl 17), as studied by 1H NMR. The reaction of 6 with 1 and of 7 with 3 did not furnish carbene products. Compounds 8–10 were characterized by ESI-MS, IR, 1D (1H, 13C{H}) and 2D (1H,1H–COSY, 1H,13C-HSQC, 1H,13C-HMBC) NMR spectroscopic techniques, and, only for 8, elemental analyses (C, H, N), while compounds 11–17 were characterized by 1D (1H, 13C{H}) and 2D (1H,13C-HSQC) NMR. Structures of compounds 8, 9, and 13 were additionally established by single-crystal X-ray diffraction.