A.P. Molchanov, R.S. Savinkov, A.V. Stepakov, G.L. Starova, R.R. Kostikov, V.S. Barnakova, A.V. Ivanov
“A Highly Efficient and Stereoselective Cycloaddition of Nitrones to N-Vinylpyrroles”
Synthesis, 2014, 46, 0771-0780
DOI: 10.1055/s-0033-1340479
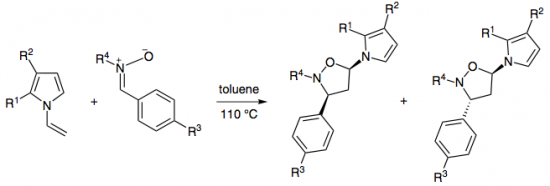
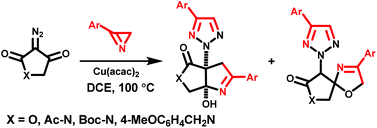
1,3-Dipolar cycloadditions of a number of C-aryl, C-carbamoyl-, and C,C-bis(methoxycarbonyl)nitrones and substituted N-vinylpyrroles proceed with high efficiency and regioselectivity with the formation of only one isomeric substituted 5-(1H-pyrrol-1-yl)isoxazolidine cycloadduct.
A.V. Stepakov, A.G. Larina, V.M. Boitsov, V.V. Gurzhiy, A.P. Molchanov, R.R. Kostikov
“Synthesis of indene derivatives via reactions of vinylidenecyclopropanes with the N-acyliminium cations generated from hydroxylactams”
Tetrahedron Lett., 2014, 55, 2022-2026
DOI: 10.1016/j.tetlet.2014.02.039
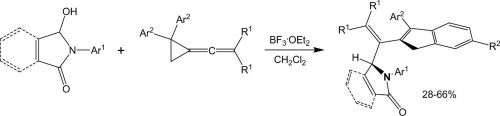
A novel route for the synthesis of 1H-indene derivatives via the reactions of vinylidenecyclopropanes (VCPs) with the N-acyliminium cations generated from hydroxylactams is described.