I.O. Koshevoy, Y.-C. Chang, Y.-A. Chen, A.J. Karttunen, E.V. Grachova, S.P. Tunik, J.Janis, T.A. Pakkanen, P.-T. Chou
“Luminescent Gold(I) Alkynyl Clusters Stabilized by Flexible Diphosphine Ligands”
Organometallics, 2014, ASAP
DOI: 10.1021/om5002952
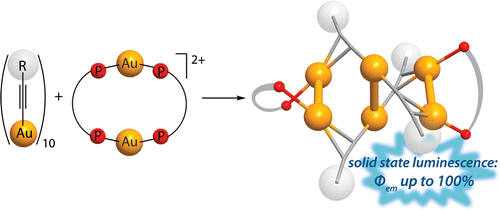
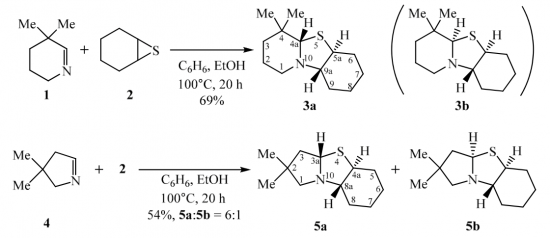
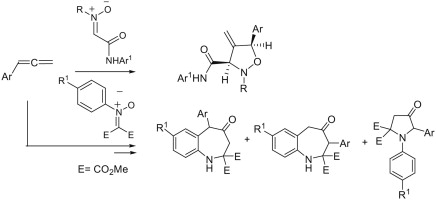
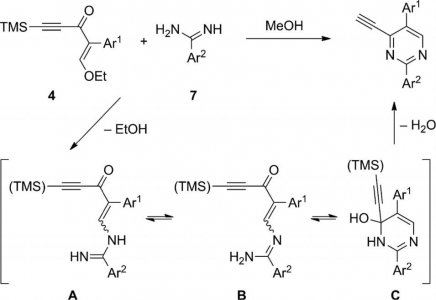
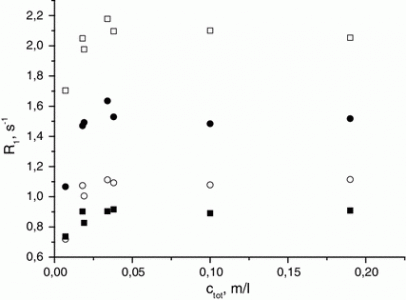
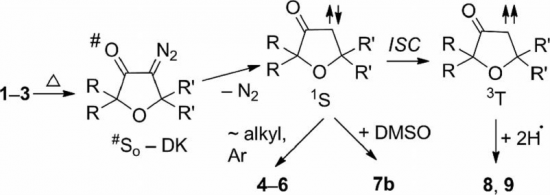
Treatment of the homoleptic decanuclear compounds (AuC2R)10 with the cationic gold diphosphine complexes [Au2(PR′2-X-PR′2)2]2+ results in high-yield formation of the new family of hexanuclear clusters [Au6(C2R)4(PR′2-X-PR′2)2]2+ (PR′2-X-PR′2 = PPh2-(CH2)n-PPh2, n = 2 (1, R = diphenylmethanolyl), n = 3 (3, R = diphenylmethanolyl; 4, R = 1-cyclohexanolyl; 5, R = 2-borneolyl), 4 (6, R = 1-cyclohexanolyl); PR′2-X-PR′2 = PCy2-(CH2)2-PCy2 (2, R = diphenylmethanolyl); PR′2-X-PR′2 = 1,2-(PPh2-O)-C6H4 (7, R = diphenylmethanolyl); PR′2-X-PR′2 = (R,R)-DIOP (8, R = diphenylmethanolyl)). In the case of PPh2-(CH2)4-PPh2 phosphine and −C2C(OH)Ph2 alkynyl ligands an octanuclear cluster of a different structural type, [Au8(C2C(OH)Ph2)6(PPh2-(CH2)4-PPh2)2]2+ (9), was obtained. Complexes 1–3, 7, and 9 were studied by X-ray crystallography. NMR and ESI-MS spectroscopic investigations showed that all but two (2 and 9) compounds are fluxional in solution and demonstrate dissociative chemical equilibria between major and a few minor forms. All of these complexes are intensely emissive in the solid state at room temperature and demonstrate very high quantum yields from 0.61 to 1.0 with weak influence of the alkynyl substituents R′ and the diphosphine backbones on luminescence energies. Two crystalline forms of the cluster 2 (P21/n and P21 space groups) exhibit unexpectedly contrasting yellow and sky blue emission, maximized at 572 and 482 nm, respectively. Electronic structure calculations with density functional methods demonstrate that the transitions responsible for the highly effective phosphorescence are dominated by contributions from the Au and π-alkynyl orbitals.