O.Yu. Bakulina, A.Yu. Ivanov, P.S. Lobanov, D.V. Dar’in
“Synthesis of novel peri-fused heterocyclic systemsdpyrimido [4,5,6-de][1,8]naphthyridines, based on interaction of 4,6-dichloro-2-methylthiopyrimidine-5-carbaldehyde with geminal enediamines”
Tetrahedron , 2014, 70, 7900-7905
DOI:10.1016/j.tet.2014.08.066
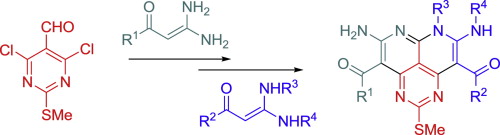
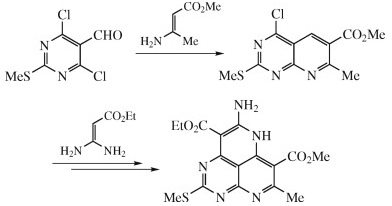
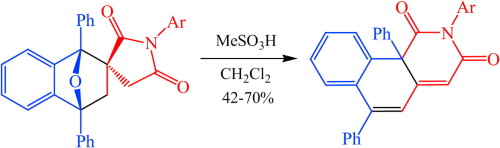
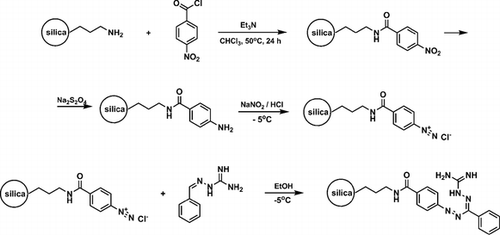
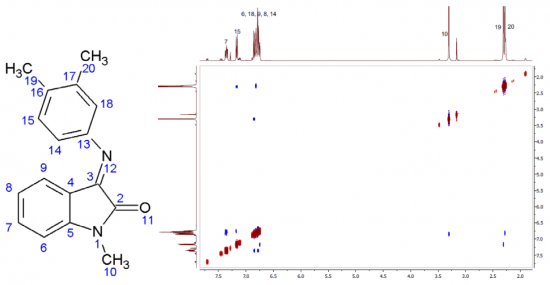
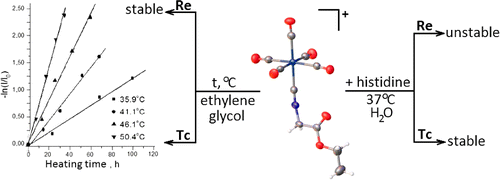
The synthetic approach to novel peri-fused heterocyclic systems—pyrimido[4,5,6-de][1,8]naphthyridines, has been developed. It consists of successive treatment of 4,6-dichloro-2-methylthiopyrimidine-5-carbaldehyde with 2 mol of geminal β-(acyl)enediamines and includes substitution of a chlorine atom with the nucleophilic carbon atom of the enediamine and cyclization of the corresponding intermediate.