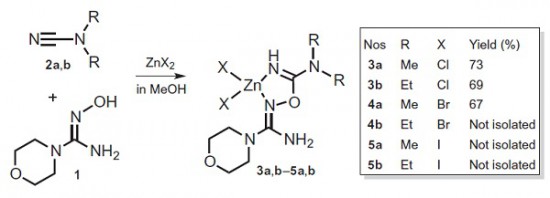
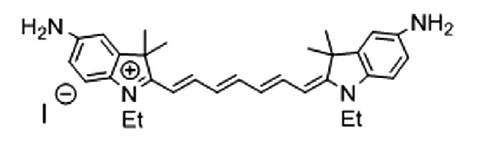
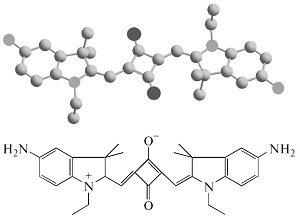
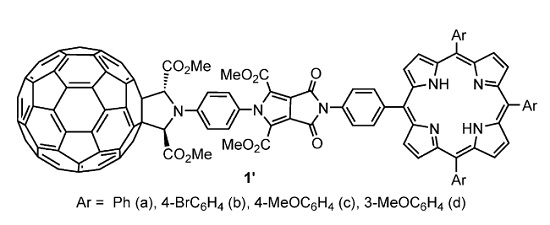
A.S. Konev, A.F. Khlebnikov, P.I. Prolubnikov, A.S. Mereshchenko, A.V. Povolotskiy, O.V. Levin, A. Hirsch, “Synthesis of New Porphyrin–Fullerene Dyads Capable of Forming Charge-Separated States on a Microsecond Lifetime Scale”
Chem. Eur. J. 2014, 20, 1-15
DOI: 10.1002/chem.201404435
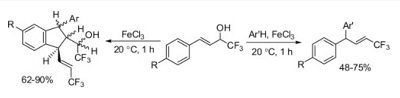
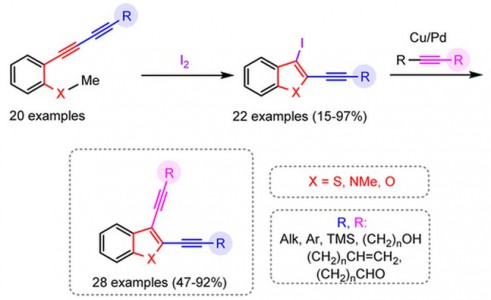
N.A. Danilkina, A.E. Kulyashova, A.F. Khlebnikov, S. Bräse, I.A. Balova, “Electrophilic Cyclization of Aryldiacetylenes in the Synthesis of Functionalized Enediynes Fused to a Heterocyclic Core”
J. Org. Chem. 2014, 79(19), 9018-9045
DOI: 10.1021/jo501396s
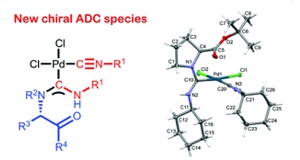
A.F. Khlebnikov, O.A. Tomashenko, L.D. Funt, M.S. Novikov, “Simple Approach to Pyrrolylimidazole Derivatives by Azirine Ring Expansion with Imidazolium Ylides”
Org. Biomol. Chem. 2014, 12, 6598-6609
DOI: 10.1039/c4ob00865k
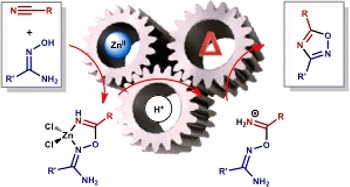
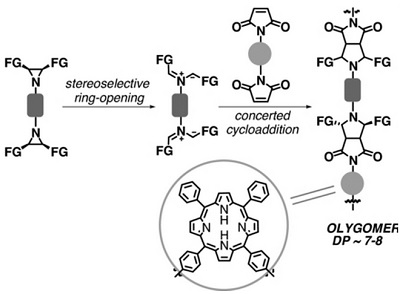
A.S. Konev, D.A. Lukyanov, P.S. Vlasov, O.V. Levin, A.A. Virtsev, I.M. Kislyakov, A.F. Khlebnikov, “The Implication of 1,3-Dipolar Cycloaddition of Azomethine Ylides to the Synthesis of Main-Chain Porphyrin Oligomers”
Macromol. Chem. Phys. 2014, 215, 516-529
DOI: 10.1002/macp.201300679
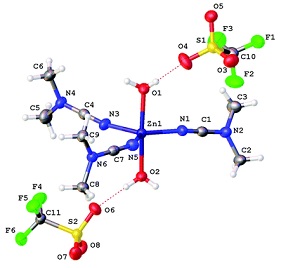
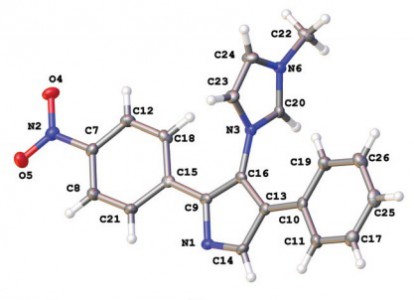
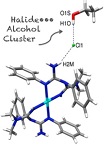
P.V. Gushchin, M.L. Kuznetsov, M. Haukka, V.Yu. Kukushkin, “Anionic halide…alcohol clusters in the solid state”
J. Phys. Chem. A 2014, 118,, 9529-9539
DOI: 10.1021/jp506256a
A.F. Khlebnikov, M.S. Novikov, Y.G. Gorbunova, E.E. Galenko, K.I. Mikhailov, V.V. Pakalnis, M.S. Avdontceva “Isoxazolium N-ylides and 1-oxa-5-azahexa-1,3,5-trienes on the way from isoxazoles to 2H-1,3-oxazines”
Beilstein J. Org. Chem. 2014, 10, 1896-1905
DOI: 0.3762/bjoc.10.197
K.V. Zavyalov, M.S. Novikov, A.F. Khlebnikov, V.V. Pakalnis, “Selective syntheses of 2H-1,3-oxazines and 1H-pyrrol-3(2H)-ones via temperature-dependent Rh(II)-carbenoid-mediated 2H-azirine ring expansion”
Tetrahedron 2014 2014, 70(21), 3377-3384
DOI: 10.1002/macp.201300679