
Svetlana Pylaeva has quit our Center. We wish her all the best at her new job!
Archive for A. Gurinov
One less again
Visit 16.05.2014

The 1st year master students of the chemistry department have visited our Center today to get familiar with NMR.
J. Molec. Struct. 2014
E.Yu. Bulatov, T.G. Chulkova, I.A. Boyarskaya, V.V. Kondratiev, M. Haukka, V.Yu. Kukushkin
“Triple associates based on (oxime)Pt(II) species, 18-crown-6, and water: Synthesis, structural characterization, and DFT study”
J. Molec. Struct. 2014, 1068, 176-181
DOI: 10.1016/j.molstruc.2014.04.010
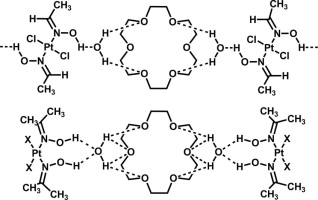
The associates 2(cis-[PtCl2(acetoxime)2])⋅18-crown-6⋅2H2O (1), 2(cis-[PtBr2(acetoxime)2])⋅18-crown-6⋅2H2O (2), and trans-[PtCl2(acetaldoxime)2]⋅(18-crown-6)⋅2H2O (3) were synthesized by co-crystallization of free corresponding platinum species and 18-crown-6 from wet solvents and characterized by 1H NMR and IR spectroscopies, high-resolution mass-spectrometry (ESI), TG/DTA, and X-ray crystallography. The (oxime)Pt(II) species are assembled with 18-crown-6 and water by hydrogen bonding between the hydroxylic hydrogen atoms of the oxime ligands and the oxygen atom of water and between the hydrogen atoms of water and the oxygen atoms of 18-crown-6. In 2(cis-[PtX2(acetoxime)2])⋅18-crown-6⋅2H2O (where X = Cl (1), Br (2)), the molecule of the crown ether is located between the two cis-[PtX2(acetoxime)2] species. The associate trans-[PtCl2(acetaldoxime)2]⋅(18-crown-6)⋅2H2O (3) crystallizes into a 1D array structure. Water molecules play the role of linkers between the (oxime)Pt(II) species and the crown ether molecules. The electronic structures and vibrational frequencies of the triple associates were studied by density functional theory (DFT/B3LYP).
Organometallics 2014
I.O. Koshevoy, Y.-C. Chang, Y.-A. Chen, A.J. Karttunen, E.V. Grachova, S.P. Tunik, J.Janis, T.A. Pakkanen, P.-T. Chou
“Luminescent Gold(I) Alkynyl Clusters Stabilized by Flexible Diphosphine Ligands”
Organometallics, 2014, ASAP
DOI: 10.1021/om5002952
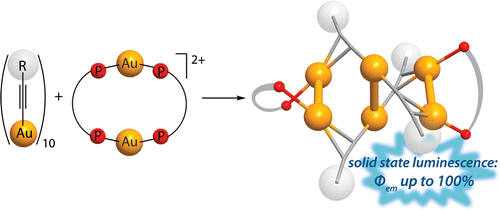
Treatment of the homoleptic decanuclear compounds (AuC2R)10 with the cationic gold diphosphine complexes [Au2(PR′2-X-PR′2)2]2+ results in high-yield formation of the new family of hexanuclear clusters [Au6(C2R)4(PR′2-X-PR′2)2]2+ (PR′2-X-PR′2 = PPh2-(CH2)n-PPh2, n = 2 (1, R = diphenylmethanolyl), n = 3 (3, R = diphenylmethanolyl; 4, R = 1-cyclohexanolyl; 5, R = 2-borneolyl), 4 (6, R = 1-cyclohexanolyl); PR′2-X-PR′2 = PCy2-(CH2)2-PCy2 (2, R = diphenylmethanolyl); PR′2-X-PR′2 = 1,2-(PPh2-O)-C6H4 (7, R = diphenylmethanolyl); PR′2-X-PR′2 = (R,R)-DIOP (8, R = diphenylmethanolyl)). In the case of PPh2-(CH2)4-PPh2 phosphine and −C2C(OH)Ph2 alkynyl ligands an octanuclear cluster of a different structural type, [Au8(C2C(OH)Ph2)6(PPh2-(CH2)4-PPh2)2]2+ (9), was obtained. Complexes 1–3, 7, and 9 were studied by X-ray crystallography. NMR and ESI-MS spectroscopic investigations showed that all but two (2 and 9) compounds are fluxional in solution and demonstrate dissociative chemical equilibria between major and a few minor forms. All of these complexes are intensely emissive in the solid state at room temperature and demonstrate very high quantum yields from 0.61 to 1.0 with weak influence of the alkynyl substituents R′ and the diphosphine backbones on luminescence energies. Two crystalline forms of the cluster 2 (P21/n and P21 space groups) exhibit unexpectedly contrasting yellow and sky blue emission, maximized at 572 and 482 nm, respectively. Electronic structure calculations with density functional methods demonstrate that the transitions responsible for the highly effective phosphorescence are dominated by contributions from the Au and π-alkynyl orbitals.
J. Organomet. Chem. 2014
A.A. Melekhova, D.V. Krupenya, V.V. Gurzhiy, A.S. Melnikov, P.Yu. Serdobintsev, S.I. Selivanov, S.P. Tunik
“Synthesis, characterization, luminescence and non-linear optical properties of diimine platinum(II) complexes with arylacetylene ligands”
J. Organomet. Chem., 2014, accepted
DOI: 10.1016/j.jorganchem.2014.04.002
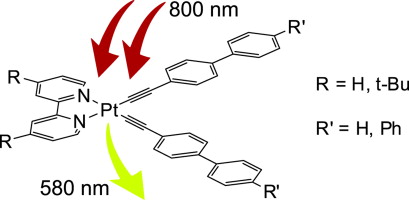
A series of platinum(II) complexes (diimine)Pt(C≣CR)2, where diimine is 2,2′-bipyridine (bpy) or 4,4′-bis(tert-butyl)-2,2′-bipyridine (dtbpy) and alkynyl ligand is biphenylacetylide or terphenylacetylide, were synthesized and their photophysical and non-linear optical properties were investigated. All compounds (1–3) were characterized using NMR spectroscopy, ESI mass-spectrometry and elemental analysis. X-Ray crystal structure of the complex containing 4,4′-bis(tert-butyl)-2,2′-bipyridine and terphenylacetylide ligands is reported. The electronic absorption and emission spectra of the complexes have been studied. Room temperature phosphorescence was observed for the complexes in solution with the luminescence quantum yield in the 7.5–11% range and excited state lifetimes in microsecond time domain. All complexes under study exhibit two-photon luminescence and their double quantum absorption cross-section was found to be in the 9–22 GM range.
Excursion 05.05.2014
Today a group of students from the University of Leiden, The Netherlands, visited our Center. The guests got acquainted with the equipment and the principles of work of the Center.
April 2014
Total in April 1700 service applications were carried out.
All together measured:
- 1631 1H spectra
- 363 13C spectra
- 193 DEPT spectra
- 39 COSY spectra
- 37 NOESY spectra
- 96 31P spectra
- 89 19F spectra
115 applications were carried out which jointly took 1370 hours of measurements.
Timetable during May 2014 holidays
30 April – shortened working day
1 – 4 May – holidays
8 May – shortened working day
9 – 11 May – holidays
User’s thesis defence
Scientific supervisor – Valery Nikolaev.
We wish the applicant to defend successfully.
Defence will take place 26.06.2014 at 15:00 at Sredniy pr. 41/43, Department of Chemistry (BCA).

