
Today Andrey Gurinov quit the Center. We wish him all the best on his is new job!

Today Andrey Gurinov quit the Center. We wish him all the best on his is new job!
Dz.N. Konshina, A.V. Furina, Z.A. Temerdashev, A.A. Gurinov, V.V. Konshin
“Immobilization of Guanazyl Functional Groups on Silica for Solid-Phase Extraction of Metal Ions”
Analytical Lett. 2014, accepted
DOI: 10.1080/00032719.2014.917421
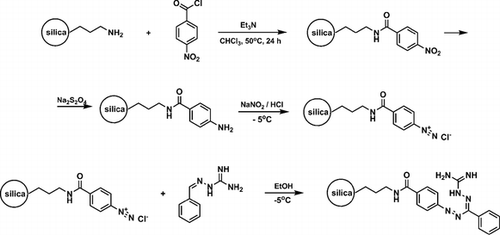
Guanazyl groups were grafted on silica gel by 4-nitrobenzoyl chloride acylation, sodium dithionite reduction, diazotation, and reaction with 2-benzylidenehydrazinecarboximidamide. The modified silica gel was used for separation and preconcentration of Cu(II), Ni(II), Cd(II), and Co(II). Quantitative extraction of the ions was achieved after 30 min and at the optimal pH of 7.5-8 at an initial concentration of 2 mg L−1. Analysis of metal sorption isotherms allowed estimation of the sorbent’s interaction efficacy under static conditions at optimal pH. Distribution coefficients were determined to be 3 ± 0.3 L g−1 for Ni(II), 3 ± 0.3 L g−1 for Co(II), 1.6 ± 0.2 L g−1 for Cd(II), and 4.6 ± 0.4 L g−1 for Cu(II) at 20-60 µg analyte. The applicability of pseudo-second order kinetic equations for metal sorption kinetics description was investigated. Chemically modified silica was used for solid-phase extraction of the metal ions to improve the detection limit using X-ray fluorescence spectrometry. The method was employed for the determination of Cu(II) in water with a low limit of detection, high accuracy, and good precision.

Today, Anastasia Kultaeva quit the Center. We wish her all the best on her new job!

Readers of “Science” column in the “Argumenty i Fakty” may learn that “The Silmarillion” is an ancient scripture, on which “The Lord of the Rings” is based; that 13 000 years ago island of Númenor drowned in the Bermuda Triangle; that the One Ring rests at the bottom of Loch-Ness where the famous monster still guarding it.
Ufa scientists led by Professor Ernst Muldashev reveal secrets of the past (Russian only).
Total in July 991 service applications were carried out.
All together measured:
68 applications were carried out which jointly took 653 hours of measurements.
P.B. Davidovich, D.S. Novikova, V.G. Tribulovich, S.N. Smirnov, V.V. Gurzhiy, G. Melino, A.V. Garabadzhiu
”First X-ray Structural Characterization of Isatin Schiff-Base Derivative. NMR and Theoretical Conformational Studies”
Journal of Molecular Structure, 2014, 1075, 450-455
DOI: 10.1016/j.molstruc.2014.07.008
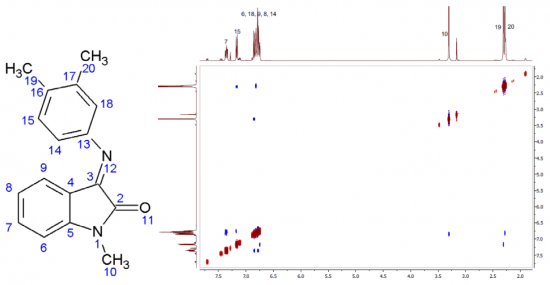
Isatin (1H-indole-2,3-dione) is an endogenous natural compound under intense development in medicinal chemistry. Here, we characterize isatin Schiff base derivative by X-ray crystallography. We describe a derivative that crystallizes E-isomer form in the triclinic space group P1; a=5.9580 (4) Å, b=8.4184 (7) Å, c=14.1801 (14) Å, α = 73.962 (8)°, β =83.184 (7)°, γ = 81.143 (6)°. NMR data show that E-conformer interconverts to the Z-conformer when dissolved, this equilibrium weakly depends on the solvent type. The Z-isomer geometry and the energetics of ΔEE-Z interconversion barriers were determined by quantum chemical calculations. The isomers are further characterized by means of FT-IR and UV-Vis spectroscopy.
A. Miroslavov, Y. Polotskii, V. Gurzhiy, A. Ivanov, A. Lumpov, M. Tyupina, G. Sidorenko, P. Tolstoy, D. Maltsev, D. Suglobov
“Technetium and Rhenium Pentacarbonyl Complexes with C2 and C11 ω-Isocyanocarboxylic Acid Esters”
Inorg. Chem. 2014, Article ASAP
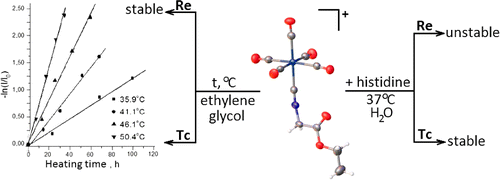
Technetium(I) and rhenium(I) pentacarbonyl complexes with ethyl 2-isocyanoacetate and methyl 11-isocyanoundecanoate, [M(CO)5(CNCH2COOEt)]ClO4 (M = Tc (1) and Re (2)) and [M(CO)5(CN(CH2)10COOMe)]ClO4 (M = Tc (3) and Re (4)), were prepared and characterized by IR, 1H NMR, and 13C{1H} NMR spectroscopy. The crystal structures of 1 and 2 were determined using single-crystal X-ray diffraction. The kinetics of thermal decarbonylation of technetium complexes 1 and 3 in ethylene glycol was studied by IR spectroscopy. The rate constants and activation parameters of this reaction were determined and compared with those for [Tc(CO)6]+. It was found that rhenium complexes 2 and 4 were stable with respect to thermal decarbonylation. Histidine challenge reaction of complexes 1 and 2 in phosphate buffer was examined by IR spectroscopy. In the presence of histidine, the rhenium pentacarbonyl isocyanide complex partially decomposes to form an unidentified yellow precipitate. Technetium analogue 1 is more stable under these conditions.