Exchange students from the University of Tokyo have visited the center.
Archive for A. Grevtsev
European Journal of Inorganic Chemistry
K.S. Kisel, G. Linti, G.L. Starova, V.V. Sizov, A.S. Melnikov, A.P. Pushkarev, M.N. Bochkarev, E.V. Grachova, S.P. Tunik
“Syntheses, Structures, and Photophysical Properties of Eu and Lu Diketonates with a Neutral Polydentate Imidazolylmethanamine Ligand”
Eur. J. Inorg. Chem., 2015
DOI: 10.1002/ejic.201403186
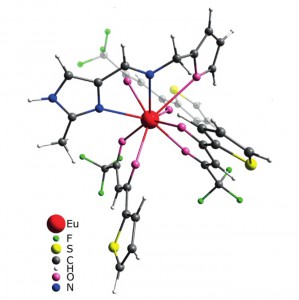
The Schiff base NNO ligand 1-(furan-2-yl)-N-[(2-methyl-1H-imidazol-4-yl)methylene]methanamine was synthesized and structurally characterized by XRD crystallography, mass spectrometry, and NMR spectroscopy. Quantum-chemical calculations revealed conformational flexibility of the ligand backbone to give two different conformations with nearly equal ground-state energies. The orientation of two nitrogen atoms and the oxygen atom in one conformation is a good fit for the NNO tridentate coordination mode, whereas the other would allow the NN coordination mode only. Two lanthanide complexes [Ln(tta)3(NNO)] (Ln = EuIII and LuIII; tta = thenoyltrifluoroacetone) were prepared and studied spectroscopically. The structures of the complexes were optimized by the DFT approach. The NNO ligand in the Eu complex displays tridentate NNO coordination, whereas the ligand is only NN-coordinated in the Lu complex. The Eu complex shows bright red metal-centered phosphorescence under excitation into the ligand (π–π*) absorption bands with a quantum yield of ca. 80 % and a lifetime of 580 μs. A mechanism for energy transfer between the ligands and metal centers was suggested and confirmed by DFT and time-dependent DFT (TDDFT) studies. An organic light-emitting diode (OLED) device based on the Eu complex incorporated into a poly(9-vinylcarbazole) (PVK) matrix was prepared. A study of the characteristics of the device revealed electrochromism of the system owing to variations in the efficacy of metal-centered and matrix emission at different strengths of applied electric field.
Beilstein J. Org. Chem. 2015
N.V. Rostovskii, M.S. Novikov, A.F. Khlebnikov, G.L. Starova, M.S. Avdontseva
“Azirinium ylides from α-diazoketones and 2H-azirines on the route to 2H-1,4-oxazines: three-membered ring opening vs 1,5-cyclization”
Beilstein J. Org. Chem., 2015, 11, 302-312
DOI: 10.3762/bjoc.11.35
Strained azirinium ylides derived from 2H-azirines and α-diazoketones under Rh(II)-catalysis can undergo either irreversible ring opening across the N–C2 bond to 2-azabuta-1,3-dienes that further cyclize to 2H-1,4-oxazines or reversibly undergo a 1,5-cyclization to dihydroazireno[2,1-b]oxazoles. Dihydroazireno[2,1-b]oxazoles derived from 3-aryl-2H-azirines and 3-diazoacetylacetone or ethyl diazoacetoacetate are able to cycloadd to acetyl(methyl)ketene generated from 3-diazoacetylacetone under Rh(II) catalysis to give 4,6-dioxa-1-azabicyclo[3.2.1]oct-2-ene and/or 5,7-dioxa-1-azabicyclo[4.3.1]deca-3,8-diene-2-one derivatives. According to DFT calculations (B3LYP/6-31+G(d,p)), the cycloaddition can involve two modes of nucleophilic attack of the dihydroazireno[2,1-b]oxazole intermediate on acetyl(methyl)ketene followed by aziridine ring opening into atropoisomeric oxazolium betaines and cyclization. Azirinium ylides generated from 2,3-di- and 2,2,3-triaryl-substituted azirines give rise to only 2-azabuta-1,3-dienes and/or 2H-1,4-oxazines.
Bowling Green State University Visitors
February
Total in February 1331 service applications were carried out.
All together measured:
- 1268 1H spectra
- 265 13C spectra
- 137 DEPT spectra
- 26 COSY spectra
- 9 NOESY spectra
- 41 31P spectra
- 53 19F spectra
156 applications were carried out which jointly took 1074 hours of measurements.
Organic & Biomolecular Chemistry
J.J. Medvedev, O.S. Galkina, A.A. Klinkova, D.S. Giera, L. Hennig, C. Schneider, V.A. Nikolaev
“Domino [4 + 1]-annulation of α,β-unsaturated δ-amino esters with Rh(II)–carbenoids – a new approach towards multi-functionalized N-aryl pyrrolidines”
Org. Biomol. Chem., 2015, 13, 2640-2651
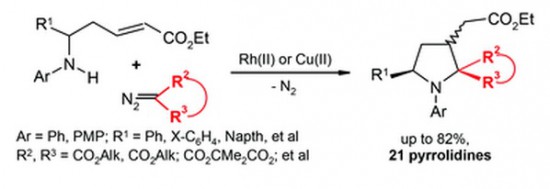
Catalytic decomposition of diazomalonates and other diazoesters using Rh(II)- and Cu(II)-complexes in the presence of α,β-unsaturated δ-(N-aryl)amino esters gives rise to the formation of multi-functionalized pyrrolidines with yields of up to 82%. The reaction apparently occurs as a domino process involving the initial N-ylide formation followed by intramolecular Michael addition to the conjugated system of amino esters to afford the pyrrolidine heterocycle. The whole process can also be classified as a [4 + 1]-annulation of the δ-amino α,β-unsaturated ester with the carbenoid intermediate.
Acta Crystallographica C 2015, C71, 155-158
D. Boyarskaya, M. Avdontceva, T. Chulkova
“Synthesis and crystal structure of 2-isocyano-4-methylphenyl diphenylacetate: a rare case of an easily accessible odourless isocyanide”
Acta Cryst. C, 2015, C71, 155-158
DOI: 10.1107/S2053229615001588
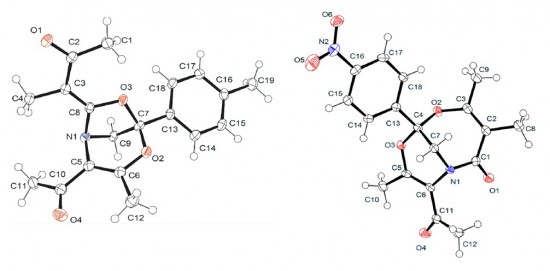
Acidic hydrogen containing 2-isocyano-4-methylphenyl diphenylacetate, C22H17NO2, (I), was synthesized by the base-promoted reaction between 5-methylbenzoxazole and diphenylacetyl chloride. Achiral (I) crystallizes in the chiral P212121 space group. The C[triple-bond]N bond length is 1.164 (2) Å and the angle between the OCO and 2-isocyano-4-methylphenyl planes is 69.10 (16)°. Molecules are linked via C=O…Hphenyl and bifurcated N[triple-bond]C…Hphenyl/N[triple-bond]C…Hmethine hydrogen bonds, forming one-dimensional arrays.
Organic & Biomolecular Chemisty 2015, 13, 1333-1338
A.S. Bogachenkov, A.V. Dogadina, V.P. Boyarskiy, A.V. Vasilyev
“Acid-promoted transformations of 1-(diphenylphosphoryl)allenes: synthesis of novel 1,4-dihydrophosphinoline 1-oxides”
Org. Biomol. Chem., 2015, 13, 1333-1338
DOI: 10.1039/c4ob02269f
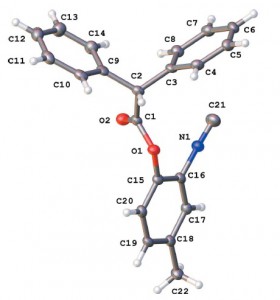
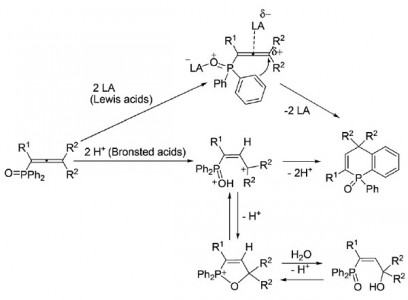
1-(Diphenylphosphoryl)alka-1,2-dienes (phosphonoallenes) in Brønsted (super)acids (TfOH, FSO3H, and H2SO4) at −70 to 120 °C for 30 min to 4 h gave, at first, (3-hydroxyalk-1-en-1-yl)diphenylphosphine oxides, as kinetically favorable reaction products, that are further converted into 1-phenyl-1,4-dihydrophosphinoline 1-oxides as thermodynamically stable compounds. The latter compounds are formed from phosphonoallenes under the action of a strong Lewis acid AlCl3 at room temperature for 10–120 min. This is a novel, simple and efficient (short reaction time, high yields) method for synthesis of such 1,4-dihydrophosphinoline 1-oxides.
January
Total in January 820 service applications were carried out.
All together measured:
- 792 1H spectra
- 198 13C spectra
- 117 DEPT spectra
- 11 COSY spectra
- 14 NOESY spectra
- 21 31P spectra
- 35 19F spectra
77 applications were carried out which jointly took 2355 hours of measurements.
Materials Science and Engineering C, 2015
A.K. Sánchez Lafarga, F.P. Pacheco Moisés, A. Gurinov, G.G. Ortiz, G.G. Carbajal Arízaga
“Dual responsive dysprosium-doped hydroxyapatite particles and toxicity reduction after functionalization with folic and glucuronic acids”
Mat. Sci. Eng. C, 2015, 48, 541-547
DOI: 10.1016/j.msec.2014.12.033
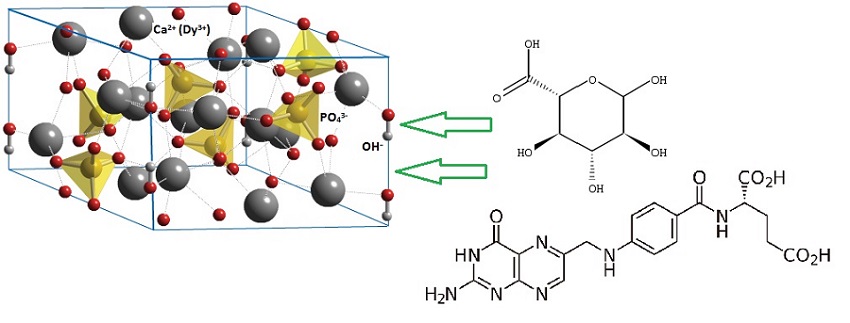
The development of probes for biomedical applications demands materials with low toxicity levels besides fluo-rescence or magnetic properties to be detected by confocal microscopes or MRI resonators. Several drug delivery systems or other biomedical