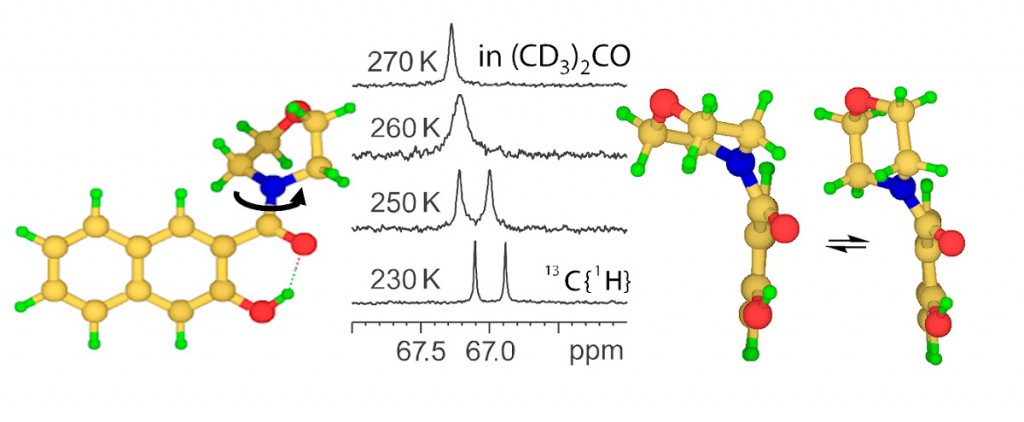
A postdoc in Prof. Dr. Nikolay R. Skrynnikov’s BioNMR laboratory, Kerstin Kampf, is researching temperature dependence of dynamics in intrinsically disordered proteins.
Archive for A. Grevtsev
Russ. J. Org. Chem. 2015, 51, 368-372
A.P. Molchanov, Yu.V. Malinina, R.R. Kostikov, A.V. Stepakov
“Regioselective Cycloaddition of C,N-Diarylnitrones to Arylallenes and of N-Aryl-C-carbamoylnitrones to Methyl Buta-2,3-dienoate”
Russ. J. Org. Chem, 2015, 51, 368-372
DOI:10.1134/S1070428015030136
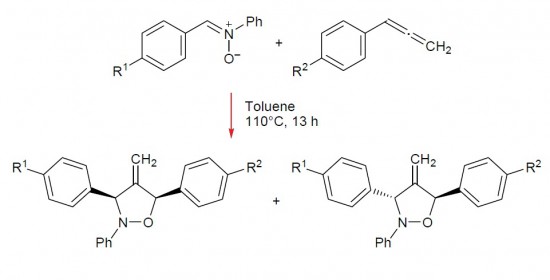
Cycloadditions of C,N-diarylnitrones to non-activated arylallenes and of N-aryl-C-carbamoylnitrones to methyl buta-2,3-dienoate regioselectively afforded mixtures of diastereoisomeric substituted 4-methylideneisoxazolidines.
Russ. J. Org. Chem. 2015, 51, 210-213
A.V. Stepakov, A.G. Larina, A.P. Molchanov
“Isomerization of Dimethylenecyclopropanes in Benzofulvenes in the Presence of Lewis Acids”
Russ. J. Org. Chem, 2015, 51, 210-213
DOI:10.1134/S1070428015020128
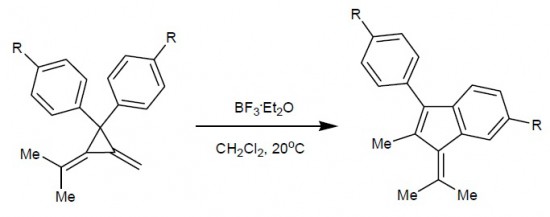
1,1-Diaryl-2-(1-methylethylidene)-3-methylenecyclopropanes in the presence of Lewis acid isomerize in benzofulvene derivatives.
Spectrochimica Acta, Part A 2015
T. Kozlecki, P.M. Tolstoy, A. Kwocz, M.A. Vovk, A. Kochel, I. Polowczyka, P.Yu. Tretyakov, A. Filarowski
“Conformational state of β-hydroxynaphthylamides and barriers for the rotation of the amide group”
Spectrochimica Acta, Part A, 2015, accepted
Three β-hydroxynaphthylamides (morpholine, pyrrolidine and dimethylamine derivatives) have been synthesized and their conformational state was analysed by NMR, X-ray and DFT calculations. In aprotic solution the molecules contain intramolecular OHO hydrogen bonds, which change into intermolecular ones in solid state. The energy barriers for the amide group rotation around the CN bond were estimated from the line shape analysis of 1H and 13C NMR signals. A tentative correlation between the barrier height and the strength of OHO bond was proposed. Calculations of the potential energy profiles for the rotations around CC and CN bonds were done. In case of morpholine derivative experimental indications of additional dynamics: chair-chair ‘ring flip’ in combination with the twisting around CC bond were obtained and confirmed by quantum chemistry calculations.
March
Total in March 1988 service applications were carried out.
All together measured:
- 1871 1H spectra
- 400 13C spectra
- 216 DEPT spectra
- 41 COSY spectra
- 11 NOESY spectra
- 88 31P spectra
- 93 19F spectra
212 applications were carried out which jointly took 2540 hours of measurements.
Students of Physics Department

As part of the course “Experimental methods in NMR” students of Physics Department have reviewed a modern scientific equipment in our center.
Dalton Trans. 2015, 44, 6003-6011
T.V. Serebryanskaya, A.S. Novikov, P.V. Gushchin, A.A. Zolotarev, V.V. Gurzhiy, V.Yu. Kukushkin
“Coupling of platinated triguanides with platinumactivated nitriles as a novel strategy for generation of dimetallic systems”
Dalton Trans., 2015, 44, 6003-6011 DOI:10.1039/c4dt03870c
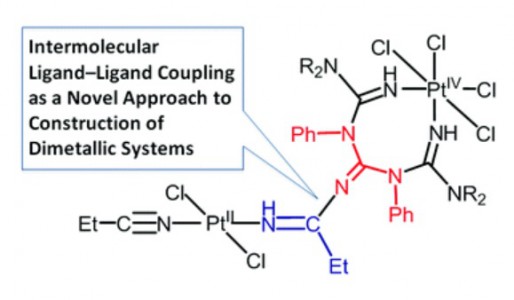
One of two PtIV-activated propanenitriles in trans-[PtCl4(EtCN)2] is involved in platinum(IV)-mediated nitrile–imine coupling with the platinum(II)-based metallacycles [PtCl2{NH[double bond, length as m-dash]C(NR2)N(Ph)C([double bond, length as m-dash]NH)N(Ph)C(NR2)[double bond, length as m-dash]NH}] [R2 = Me2 (1a), C5H10 (1b)] yielding diplatinum products, whose structures depend on molar ratios between the reactants. At a 1:1 ratio, the mixed-valence platinum(II)/platinum(IV) species [PtCl4{NH[double bond, length as m-dash]C(NR2)N(Ph)C{[double bond, length as m-dash][(N(Et)C[double bond, length as m-dash]NH)PtCl2(EtCN)]}N(Ph)C(NR2)[double bond, length as m-dash]NH}] [R2 = Me2 (2a), (CH2)5 (2b)] were generated, whereas at a 1:2 ratio the dinuclear platinum(II)/platinum(II) complexes [PtCl2{NH[double bond, length as m-dash]C(NR2)N(Ph)C{[double bond, length as m-dash][(N(Et)C[double bond, length as m-dash]NH)PtCl2(EtCN)]}N(Ph)C(NR2)[double bond, length as m-dash]NH}] [R2 = Me2 (3a), (CH2)5 (3b)] were obtained. In contrast to the nitrile–imine coupling observed for the platinum(IV) dinitrile complex, the reaction between the platinum(II) congener trans-[PtCl2(EtCN)2] and any one of 1a,b gives exclusively the substituted dimetallic platinum(II)/platinum(II) products [PtCl2{NH[double bond, length as m-dash]C(NR2)N(Ph)C{[double bond, length as m-dash][(NH)PtCl2(EtCN)]}N(Ph)C(NR2)[double bond, length as m-dash]NH}] [R2 = Me2 (6a), (CH2)5 (6b)] featuring platinum-containing guanidine 1 as one of the ligands. Complexes 2a,b, 3a,b, and 6a,b were characterized by elemental analyses (C, H, N), HRESI-MS, IR, 1H NMR spectroscopy, and DTA/TG. The molecular and crystal structure of 2a·2CDCl3 was additionally studied by single-crystal X-ray diffraction. Complexes 2a,b undergo further redox transformation in solutions, and single crystals of [PtCl2{NH[double bond, length as m-dash]C(NMe2)N(Ph)C{[double bond, length as m-dash][(N(Et)C[double bond, length as m-dash]NH)PtCl2(MeCN)]}N(Ph)C(NMe2)[double bond, length as m-dash]NH}]·2CH2Cl2 (3′a·2CH2Cl2) were obtained from 2a in a CH2Cl2–MeCN–C2H4Cl2 mixture and studied by X-ray crystallography. The driving forces for the generation of diplatinum products 2 and 3 were elucidated based on a quantum-chemical study.
British J. App. Sci. Technol. 2015
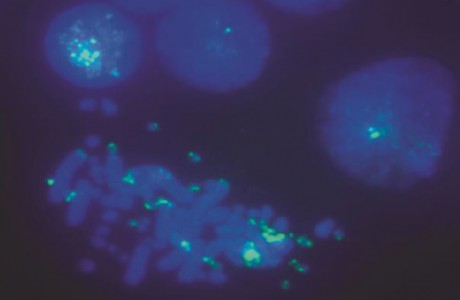
A. Stepakov, S. Galkina, D. Bogomaz, E. Gaginskaya, A. Saifitdinova
“Modified Synthesis of 6-carboxyfluorescein (6-FAM): Application to Probe Labeling for Conventional Cytogenetics”
British J. App. Sci. Technol., 2015, 7, 423-428
DOI:10.9734/BJAST/2015/15991
Aims: Fluorescent in situ hybridization (FISH), the routine technique of molecular cytogenetics, is widely used to detect and localize the presence of specific nucleic acids sequences in chromosomes, cell nucleus space, cells and tissue samples through the use of highly complementary probes to targets sequence. Expansion of FISH method application for research purposes and medical diagnostics requires efficient and low-cost production of labeled nucleic acid probes.
Methodology and Results: We developed an effective method of fluorescein hydroxyalkyl carboxamides synthesis. This modification of the basic protocol of 6-carboxyfluorescein (6-FAM) synthesis enabled the production of highly reactive conjugate perfectly suitable for terminal labeling of newly generated oligonucleotides. Efficiency of 6-FAM labeled oligonucleotides obtained by the use of modified protocol has been proved for conventional cytogenetics.
Conclusion: The suggested procedure of 6-FAM labeled oligonucleotides synthesis allows obtaining the high yield of directly labeled FISH probes. The introduction of this method into practice of cytogenetic studies will improve their efficiency and reduce the cost of an examination.
Tetrahedron 2015 (71) 2071-2078
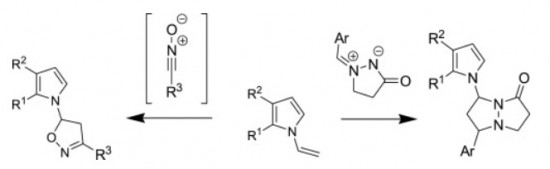
M.M. Efremova, A.P. Molchanov, A.V. Stepakov, R.R. Kostikov, V.S. Shcherbakova, A.V. Ivanov
“A highly efficient [3+2] cycloaddition of nitrile oxides and azomethine imines to N-vinylpyrroles”
Tetrahedron, 2015, 71, 2071-2078
DOI:10.1016/j.tet.2015.02.058
1,3-Dipolar cycloaddition of various nitrile oxides to substituted N-vinylpyrroles proceed with high efficiency and selectivity with the formation of single isomer of 5-pyrrolyl-substituted isoxazoline. The reaction of N-vinylpyrroles with cyclic azomethine imines occurs regioselectively affording 7-(pyrrol-1-yl)- substituted pyrazolo[1,2-a]pyrazolones as a mixture of two diastereomers.
Tetrahedron 2015 (71) 1952-1958
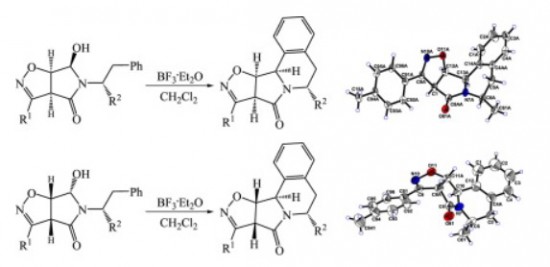
M.S. Ledovskaya, A.P. Molchanov, V.M. Boitsov, R.R. Kostikov, A.V. Stepakov
“An efficient synthesis of substituted isoxazolopyrroloisoquinolines via diastereoselective N-acyliminium ion cyclization”
Tetrahedron, 2015, 71, 1952-1958
DOI:10.1016/j.tet.2015.02.031
A simple and efficient strategy was developed for the synthesis of fused pyrrolo[2,1-a]isoquinoline ring systems. The 5- and 6-substituted isoxazolopyrroloisoquinolines were readily prepared via diastereoselective N-acyliminium ion cyclization of 5-(1-R(or 2-R)-substituted-2-phenylethyl)-6-hydroxytetrahydro-4H-pyrrolo[3,4-d]isoxazol-4-ones derived from the corresponding bicyclic dihydroisoxazoles.