For the first time at CMR Dr. Benjamin Koeppe has synthsized a mixture of deuterated freon gases (CDF3/CDF2Cl). Liquefied freons may serve as a solvent for extra low-temperature NMR experiments in liquid state (down to 100 K).
References:
1. B. Koeppe, J. Guo, P.M. Tolstoy, G.S. Denisov, H.-H. Limbach, “Solvent and H/D Isotope Effects on the Proton Transfer Pathways in Heteroconjugated Hydrogen-Bonded Phenol-Carboxylic Acid Anions Observed by Combined UV–vis and NMR Spectroscopy”, J. Am. Chem. Soc. 2013, 135, 7553-7566. DOI: 10.1021/ja400611x.
2. J. Guo, P.M. Tolstoy, B. Koeppe, N.S. Golubev, G.S. Denisov, S.N. Smirnov, H.-H. Limbach, “Hydrogen Bond Geometries and Proton Tautomerism of Homo-Conjugated Anions of Carboxylic Acids Studied via H/D Isotope Effects on 13C NMR Chemical Shifts”, J. Phys. Chem. A 2012, 116, 11180-11188. DOI: 10.1021/jp304943h.
3. D. Ajami, P.M. Tolstoy, H. Dube, S. Odermatt, B. Koeppe, J. Guo, H.-H. Limbach, J. Rebek Jr., “Compressed Hydrogen Bonds Isolated in Encapsulation Complexes”, Angew. Chem. Int. Ed. 2011, 50, 528-531. DOI: 10.1002/anie.201002182.
4. P.M. Tolstoy, P. Schah-Mohammedi, S.N. Smirnov, N.S. Golubev, G.S. Denisov, H.-H. Limbach, „Characterization of Fluxional Hydrogen-Bonded Complexes of Acetic Acid and Acetate by NMR: Geometries and Isotope and Solvent Effects”, J. Am. Chem. Soc., 2004, 126, 5621-5634. DOI: 10.1021/ja039280j.
Archive for A. Grevtsev
Solvent for extra low-temperature NMR
A. Rozentsvet has presented a lecture

On 21.05 at CMR Dr. Victor A. Rozentsvet (Institute of Ecology of the Volga River Basin, Russian Academy of Sciences, Togliatti, Russia) has presented a lecture titled “Unusual NMR spectra of branched diene polymers. Questions without answers.”
Combined acquisition of NMR and UV-vis absorption spectra
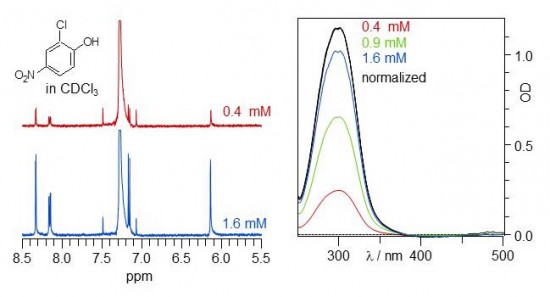
Dr. Benjamin Koeppe (Humboldt-Universität zu Berlin, Germany), who previously worked in the group of Prof. Dr. Hans-Heinrich Limbach and developed a technique for the combined acquisition of NMR and UV-vis absorption spectra (UVNMR), is installing and demonstrating the equipment at the CMR. The figure shows NMR and UV-vis spectra of 2-chloro-4-nitrophenol dissolved in chloroform, obtained simultaneously within the magnet of an NMR spectrometer..
Dr. Benjamin’s Koeppe presentation
On Monday 18.05.15 at 15:00 in the room 1093 (seminar room of X-ray diffraction center) our guest Dr. Benjamin Koeppe (HU Berlin, Germany) will give a presentation titled “Combined NMR and UV-vis studies of hydrogen bonded complexes”.
April
Total in April 1664 service applications were carried out.
All together measured:
- 1600 1H spectra
- 376 13C spectra
- 180 DEPT spectra
- 21 COSY spectra
- 4 NOESY spectra
- 36 31P spectra
- 108 19F spectra
208 applications were carried out which jointly took 1831 hours of measurements.
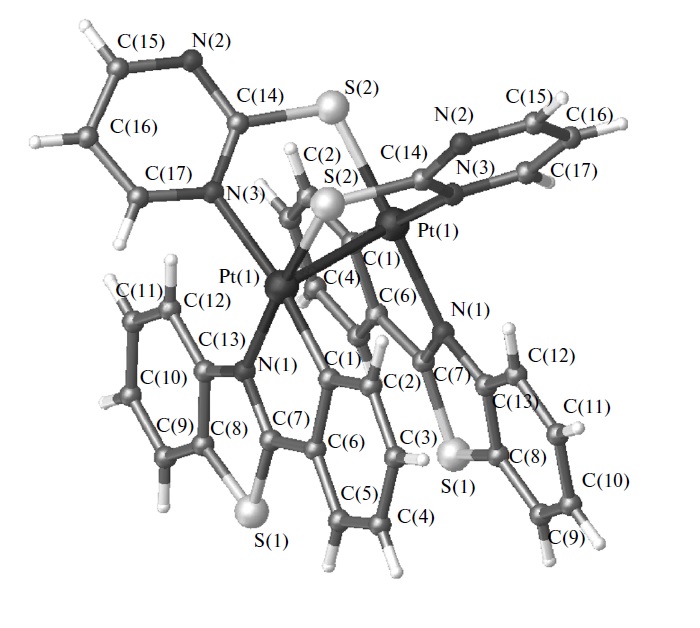
Russian Journal of Coordination Chemistry, 2015, 41(6), 357-364
E.A. Katlenok, A.A. Zolotarev, A. U. Ivanov, S.N. Smirnov, R.I. Baychurin, K.P. Balashev
Title in rus: “Cтроение, оптические и электрохимические свойства биядерных платинированных комплексов 2-фенилбензотиазола с мостиковыми 2-меркаптопроизводными тиазолина, 1-метилимидазола и пиримидина”
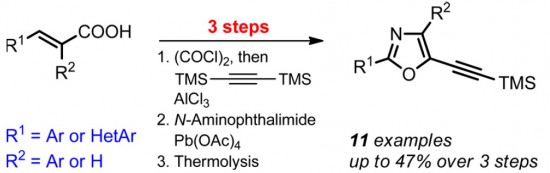
Org. Lett. 2015
A.S. Pankova, A.Yu. Stukalov, M.A. Kuznetsov
“Synthesis of 2‑(Hetero)aryl-5-(trimethylsilylethynyl)oxazoles from 2 (Hetero)arylacrylic Acids”
Org. Lett., 2015, accepted
DOI:10.1021/acs.orglett.5b00009
A three-step method for the synthesis of 2-(hetero)-aryl-5-(trimethylsilylethynyl)oxazoles is described. Easily accessible bis(trimethylsilyl)acetylene and acrylic acid derivatives are used as starting materials for the preparation of mono- and disubstituted 5-(trimethylsilyl)pent-1-en-4-yn-3-ones. Oxidative phthalimido-aziridination of these enynones provides the key 2-acyl-1-phthalimido-aziridines that are further utilized in the thermal expansion of the three-membered ring to furnish the target functionalizable oxazoles.
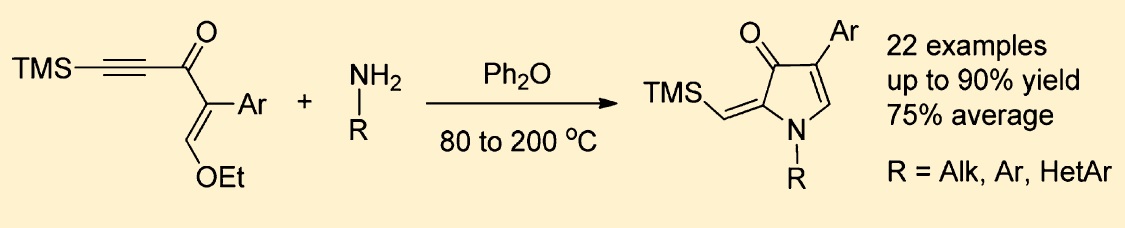
J. Org. Chem. 2015
P.R. Golubev, A.S. Pankova, M.A. Kuznetsov
“Regioselective Transition-Metal-Free Synthesis of 2‑(Trimethylsilylmethylene)pyrrol-3-ones by Thermal Cyclization of Acetylenic Enamines”
J. Org. Chem., 2015, accepted
DOI:10.1021/acs.joc.5b00398
Acetylenic enamines generated in situ from readily available enynones and primary amines undergo thermal cyclization in diphenyl ether providing easy access to 4-aryl-2-(trimethylsilylmethylene)-1,2-dihydro-3H-pyrrol-3-ones. This reaction is inherently versatile, allowing for variations of substituents in both enynone and amine. Full regioselectivity along with short reaction time (1−2 h) and simple workup afford single products in good to excellent isolated yields. Fluorescent properties of the obtained compounds were studied.>
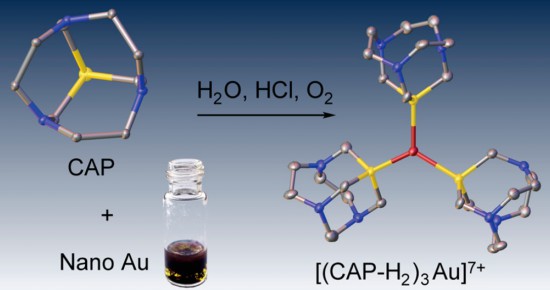
J. Am. Chem. Soc. 2015
S.N. Britvin, A. Lotnyk
“Water-Soluble Phosphine Capable of Dissolving Elemental Gold: The Missing Link between 1,3,5-Triaza-7-phosphaadamantane (PTA) and Verkade’s Ephemeral Ligand”
J. Am. Chem. Soc., 2015, accepted
DOI:10.1021/jacs.5b01851
We herein describe a tricyclic phosphine with previously unreported tris(homoadamantane) cage architecture. That water-soluble, air- and thermally stable ligand, 1,4,7-triaza-9-phosphatricyclo[5.3.2.14,9]tridecane (hereinafter referred to as CAP) exhibits unusual chemical behavior toward gold and gold compounds: it readily reduces Au(III) to Au(0), promotes oxidative dissolution of nanocrystalline gold(0) with the formation of water-soluble trigonal CAP−Au(I) complexes, and displaces cyanide from [Au(CN)2]− affording triangular [Au(CAP)3]+ cation. From the stereochemical point of view, CAP can be regarded as an intermediate between 1,3,5-triaza-7-phosphaadamantane (PTA) and very unstable aminophosphine synthesized by Verkade’s group: exahydro-2a,4a,6a-triaza-6bphosphacyclopenta[cd]pentalene. The chemical properties of CAP are likely related to its anomalous stereoelectronic profile: combination of strong electron-donating power (Tolman’s electronic parameter 2056.8 cm−1) with the low steric demand (coneangle of 109°). CAP can be considered as macrocyclic counterpart of PTA with the electron-donating power approaching that of strongest known phosphine electron donors such as P(t-Bu)3 and PCy3. Therefore, CAP as sterically undemanding and electronrich ligand populates the empty field on the stereoelectronic map of phosphine ligands: the niche between the classic tertiary phosphines and the sterically undemanding aminophosphines.
Our visitors
On April 24th 2015 Behruz Abtahi (representative of the Ministry of Science, Research and Technologies of Iran, Counsellor of the Embassy of the Iranian Republic), Mohammad Mehdi Tehranchi (Chancellor of Shahid Beheshti University, Tehran) and Kayvan Hosseini (Assistant professor at Allameh Tabatabai University, Tehran) have visited the center.