Archive for A. Grevtsev
Lecture of Dr. Tomasz Kozlecki
J. Hetercyclic Chem. 2015, 52, 1192-1194
D.V. Dar’in, А.Yu. Ivanov, P.S. Lobanov
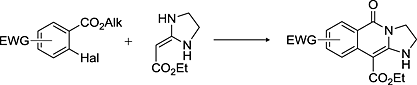
“Cyclocondensation of Ethyl (imidazolidine-2-ylidene)acetate with Aromatic Esters Bearing Labile Halogen in ortho-Position”
J. Hetercyclic Chem., 2015, 52, 1192-1194
DOI:10.1002/jhet.2229
Cyclocondensation of ethyl (imidazolidine-2-ylidene)acetate with aromatic esters bearing labile halogen in ortho-position leads to fused heterocycles, which is formed by substitution of halogen atom with α-carbon atom of cyclic ketene aminal and binding of nitrogen atom with carbonyl carbon atom of aromatic ester.
Tetrahedron 2015, 71, 6196-6203
M.E. Chizhova, O.Yu. Bakulina, A.Yu. Ivanov, P.S. Lobanov, D.V. Dar’in
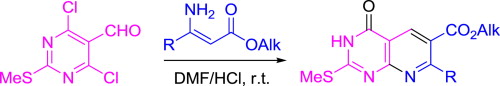
“Facile synthesis of pyrido[2,3-d]pyrimidines via cyclocondensation of 4,6-dichloro-2-methylsulfanylpyrimidine-5-carbaldehyde with β-substituted β-aminoacrylic esters”
Tetrahedron, 2015, 71, 6196-6203
DOI:10.1016/j.tet.2015.06.085
A new facile synthesis of pyrido[2,3-d]pyrimidin-4-ones via cyclocondensation of 4,6-dichloro-2-methylsulfanylpyrimidine-5-carbaldehyde with β-alkyl and β-aryl-β-aminoacrylic esters followed by hydrolysis of chlorine atom at position 4 of pyridopyrimidine ring has been developed. The cyclocondensation was found to be accelerated by acid.
Carla Pretorius has presented a lecture
On September 11th Carla Pretorius (University of the Free State, Bloemfontein, South Africa) will present a lecture “Novel 1-D rhodium polymeric chain systems via rhodium-rhodium interactions”. The seminar will take place at 17:00 at the Institute of Chemistry in the audience 1093.
Dr. Tomasz Kozlecki has presented a lecture

On September 8th Dr. Tomasz Kozlecki (Wroclaw University of Technology, Poland) will present a lecture titled “Synthesis and biological activity of selected nanoparticles”. The seminar will take place at 16:00 at the Institute of Chemistry in the audience 1093.
More information you can find here.
Visitor from Wroclaw University of Technology
MRI setup for Bruker Avance 400WB

CMR specialist A.S. Mazur with colleagues (A.V. Dobrodumov, O.M. Osmolovskaya, V.A. Korzhikov and others) are testing the MRI setup for Bruker Avance 400WB spectrometer. On screen: lab mice MR-images.
Organic & Biomolecular Chemistry 2015, 13, 8827-8842
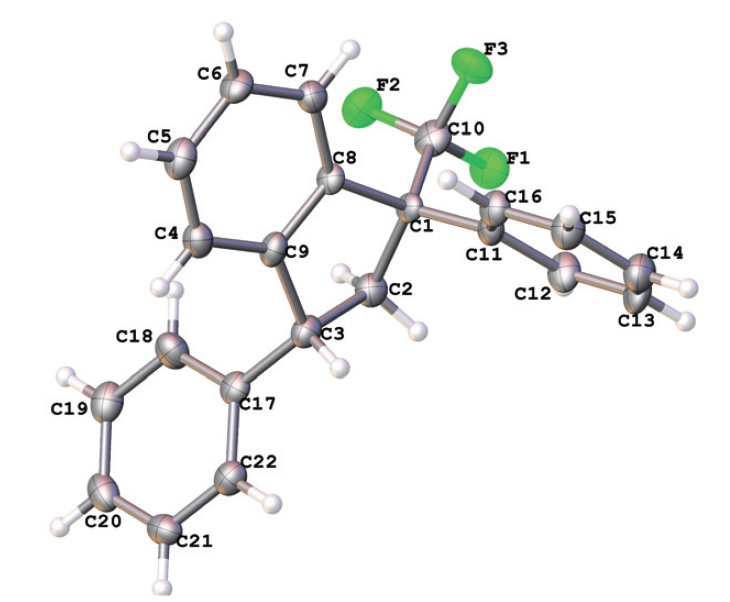
R.O. Iakovenko, A.N. Kazakova, V.M. Muzalevskiy, A.Yu. Ivanov, I.A. Boyarskaya, A. Chicca, V. Petrucci, J. Gertsch, M. Krasavin, G.L. Starova, A.A. Zolotarev, M.S. Avdontceva, V.G. Nenajdenko, A.V. Vasilyev
“Reactions of CF3-enones with arenes under superelectrophilic activation: a pathway to trans-1,3-diaryl-1-CF3-indanes, new cannabinoid receptor ligands”
Org. Biomol. Chem., 2015, 13, 8827-8842
DOI:10.1039/c5ob01072a
4-Aryl-1,1,1-trifluorobut-3-en-2-ones ArCH=CHCOCF3 (CF3-enones) react with arenes in excess of Brønsted superacids (TfOH, FSO3H) to give, stereoselectively, trans-1,3-diaryl-1-trifluoromethyl indanes in 35–85% yields. The reaction intermediates, the O-protonated ArCH=CHC(OH+)CF3 and the O,C-diprotonated ArHC+CH2C(OH+)CF3 species, have been studied by means of 1H, 13C, 19F NMR, and DFT calculations. Both types of the cations may participate in the reaction, depending on their electrophilicity and electron-donating properties of the arenes. The formation of CF3-indanes is a result of cascade reaction of protonated CF3-enones to form chemo-, regio- and stereoselectively three new C–C bonds. The obtained trans-1,3-diaryl-1-trifluoromethyl indanes were investigated as potential ligands for cannabinoid receptors CB1 and CB2 types. The most potent compound showed sub-micromolar affinity for both receptor subtypes with a 6-fold selectivity toward the CB2 receptor with no appreciable cytotoxicity toward SHSY5Y cells.