I.S. Ignatyev, T.A. Kochina, V.V. Avrorin, V.V. Gurzha, I.M. Fundamensky
“Molecular and crystal structure of 2-phenyl-2-hydro-6-methyl-1,3-dioxa-6-aza-2-silacyclooctane”
J. Molec. Struct., 2015, 1094, 169-173
DOI:10.1016/j.molstruc.2015.03.061
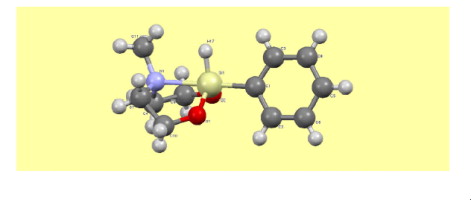
The crystal structure of 2-phenyl-2-hydro-6-methyl-1,3-dioxa-6-aza-2-silacyclooctane [HPhSi(OCH2CH2)2NMe – phenylhydrosilocane (I)] is determined by single-crystal X-ray diffraction at 100 K. The unit cell consists of four molecules connected only by Van-der-Waals interactions. Each molecule has an eight-membered heterocycle with a phenyl group in the axial position. The Si⋯N transannular bond has a short (2.206 Å) interatomic distance which exceeds only this distance in ocanes with highly electronegative fluorine substituents at Si. Since there exist experimental data on the occurrence of different conformers of I in the liquid phase, the PES of the molecule was analyzed by DFT B3LYP and MP2 methods with the aug-cc-pVDZ basis set. The energy minimum belongs to the boat–chair conformation with the axial position of the phenyl group. Rotation of the phenyl ring around the SiC bond has a barrier ca. 1 kcal/mol. The conformer with the equatorial position of this group lies 6 kcal/mol higher. Interconversion of this conformers which was observed in experiment proceeds through the chair–chair configuration in which the Si⋯N transannular bond is absent and coordination at silicon is tetrahedral, rather than trigonal bipyramidal one observed in other conformers.