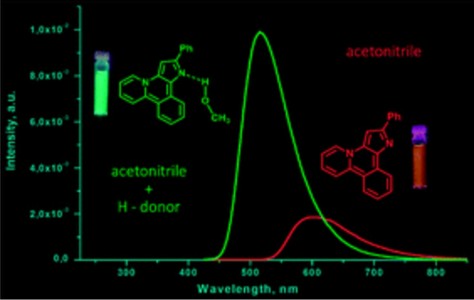
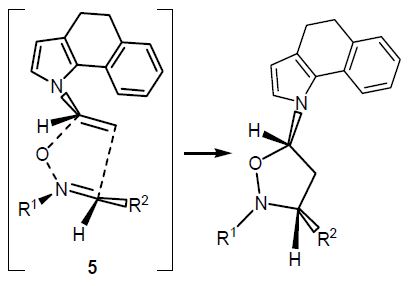
N.A. Danilkina, A.G. Lyapunova, A.F. Khlebnikov, G.L. Starova, S. Brase, I.A. Balova
“Ring-Closing Metathesis of Co2(CO)6–Alkyne Complexes for the Synthesis of 11-Membered Dienediynes: Overcoming Thermodynamic Barriers”
J. Org. Chem., 2015, 80, 5546-5555
DOI:10.1021/acs.joc.5b00409
The feasibility of ring-closing metathesis (RCM) as a synthetic entry to 10- and 11-membered dienediynes fused to a benzothiophene core was explored by experimental and theoretical investigations. An established sequence of iodocyclization of o-(buta-1,3-diynyl)thioanisoles followed by Sonogashira coupling to form diethynylbenzothiophenes was used to synthesize terminal benzothiophene-fused enediyne diolefins as substrates for RCM. Encountering an unexpected lack of reactivity of these substrates under standard RCM conditions, we applied DFT calculations to reveal that the underlying cause was a positive change in Gibbs free energy. The change in Gibbs free energy was also found to be positive for RCM of indole- and benzannulated terminal diolefins when affording smaller than 12-membered rings. We found that modification of the enediyne–diolefin substrate as the Co2(CO)6–alkyne complex allowed the target benzothiophene-fused 11-membered dienediyne to be obtained via RCM; the alkyne complexation strategy therefore provides one valid technique for overcoming challenges to macrocyclization of this kind.