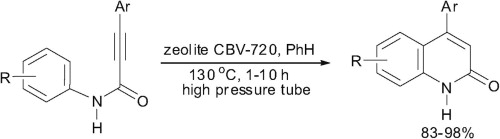
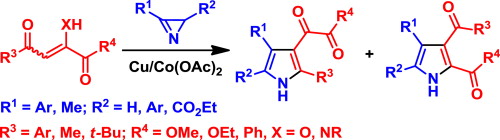
A.A. Beljaev, D.V. Krupenya, E.V. Grachova, V.V. Gurzhiy, A.S. Melnikov, P.Yu. Serdobintsev, E.S. Sinitsyna, E.G. Vlakh, T.B. Tennikova, S.P. Tunik
“Supramolecular AuI-CuI complexes as new luminescent labels for covalent bioconjugation”
Bioconjugate Chem., 2015
DOI:10.1021/acs.bioconjchem.5b00563
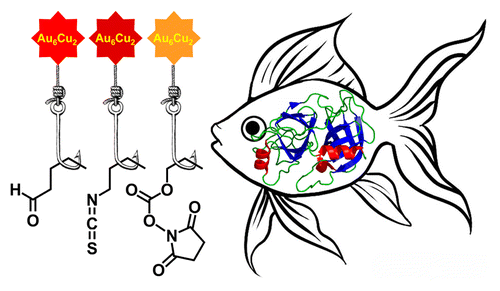
Two new supramolecular organometallic complexes, namely, [Au6Cu2(C2C6H4CHO)6(PPh2C6H4PPh2)3](PF6)2 and [Au6Cu2(C2C6H4NCS)6(PPh2C6H4PPh2)3](PF6)2, with highly reactive aldehyde and isothiocyanate groups have been synthesized and characterized using X-ray crystallography, ESI mass spectrometry, and NMR spectroscopy. The compounds obtained demonstrated bright emission in solution with the excited-state lifetime in microsecond domain both under single- and two-photon excitation. The luminescent complexes were found to be suitable for bioconjugation in aqueous media. In particular, they are able to form the covalent conjugates with proteins of different molecular size (soybean trypsin inhibitor, human serum albumin, rabbit anti-HSA antibodies). The conjugates demonstrated a high level of the phosphorescent emission from the covalently bound label, excellent solubility, and high stability in physiological media. The highest quantum yield, storage stability, and luminance were detected for bioconjugates formed by covalent attachment of the aldehyde-bearing supramolecular AuI–CuI complex. The measured biological activity of one of the labeled model proteins clearly showed that introduced label did not prevent the biorecognition and specific protein–protein complex formation that was extremely important for the application of the conjugates in biomolecular detection and imaging.