N.V. Rostovskii, M.S. Novikov, A.F. Khlebnikov, G.L. Starova, M.S. Avdontseva
“Azirinium ylides from ?-diazoketones and 2H-azirines on the route to 2H-1,4-oxazines: three-membered ring opening vs 1,5-cyclization”
Beilstein J. Org. Chem., 2015, 11, 302-312
DOI:10.3762/bjoc.11.35
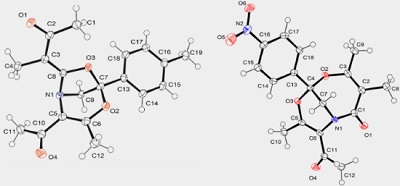
Strained azirinium ylides derived from 2H-azirines and α-diazoketones under Rh(II)-catalysis can undergo either irreversible ring opening across the N–C2 bond to 2-azabuta-1,3-dienes that further cyclize to 2H-1,4-oxazines or reversibly undergo a 1,5-cyclization to dihydroazireno[2,1-b]oxazoles. Dihydroazireno[2,1-b]oxazoles derived from 3-aryl-2H-azirines and 3-diazoacetylacetone or ethyl diazoacetoacetate are able to cycloadd to acetyl(methyl)ketene generated from 3-diazoacetylacetone under Rh(II) catalysis to give 4,6-dioxa-1-azabicyclo[3.2.1]oct-2-ene and/or 5,7-dioxa-1-azabicyclo[4.3.1]deca-3,8-diene-2-one derivatives. According to DFT calculations (B3LYP/6-31+G(d,p)), the cycloaddition can involve two modes of nucleophilic attack of the dihydroazireno[2,1-b]oxazole intermediate on acetyl(methyl)ketene followed by aziridine ring opening into atropoisomeric oxazolium betaines and cyclization. Azirinium ylides generated from 2,3-di- and 2,2,3-triaryl-substituted azirines give rise to only 2-azabuta-1,3-dienes and/or 2H-1,4-oxazines.