Archive for A. Grevtsev
Inorg. Chem. Comm. 2015, 61, 21-23
V.A. Rassadin, A.A. Yakimanskiy, E.V. Eliseenkov, V.P. Boyarskiy
“Synthesis of acyclic diaminocarbene palladium complex featuring triethoxysilane moiety”
Inorg. Chem. Comm., 2015, 2015, 61, 21-23
DOI:10.1016/j.inoche.2015.08.008
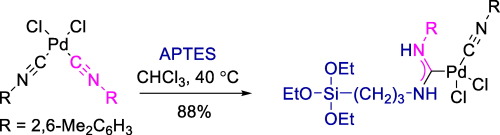
The first example of an acyclic diaminocarbene palladium complex, viz. cis-[PdCl2(CN(2,6-Me2C6H3)){C(NH(2,6-Me2C6H3)) = NH(CH2)3Si(OEt)3}], featuring the triethoxysilane moiety is described. The complex was generated from bis(o-xylylisocyanide)palladium dichloride and (3-aminopropyl)triethoxysilane under mild conditions and isolated in 88% yield. The target compound is stable at RT either in the solid state or in CDCl3 or CD3OD solutions within several months.
Febrary
Total in Febrary 1749 service applications were carried out.
All together measured:
- 1680 1H spectra
- 341 13C spectra
- 150 DEPT spectra
- 24 COSY spectra
- 15 NOESY spectra
- 73 31P spectra
- 58 19F spectra
206 applications were carried out.
Inorg. Chem., 2015, 54, 4039-4046
D.S. Bolotin, M.Ya. Demakova, A.S. Novikov, M.S. Avdontceva, M.L. Kuznetsov, N.A. Bokach, V.Yu. Kukushkin
“Bifunctional Reactivity of Amidoximes Observed upon Nucleophilic Addition to Metal-Activated Nitriles”
Inorg. Chem., 2015, 54, 4039-4046
DOI:10.1021/acs.inorgchem.5b00253
Treatment of the aromatic nitrile complexes trans-[PtCl2(RC6H4CN)2] with the aryl amidoximes p-R′C6H4C(NH2)═NOH, followed by addition of 1 equiv of AgOTf and then 5 equiv of Et3N, leads to the chelates [PtCl{HN═C(RC6H4)ON═C(C6H4R′-p)NC(RC6H4)═NH}] (15 examples; yields 71−88% after column chromatography) derived from the platinum(II)-mediated coupling between metal-activated nitriles and amidoximes. The combined experimental and theoretical results indicate that the coupling with the nitrile ligands involves both the HON and monodeprotonated NH2 groups of the amidoximes.
Org. Lett. 2015, 17, 3502-3505
V.A. Rassadin, V.P. Boyarskiy, V.Yu. Kukushkin
“Facile Gold-Catalyzed Heterocyclization of Terminal Alkynes and Cyanamides Leading to Substituted 2-Amino-1,3-Oxazoles”
Org. Lett., 2015, 17, 3502-3505
DOI:10.1021/acs.orglett.5b01592
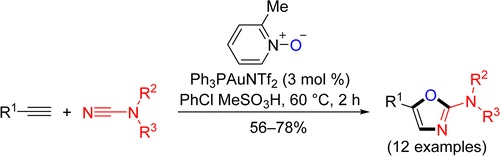
Facile gold-catalyzed heterocyclization based upon intermolecular trapping of the generated α-oxo gold carbenes with various cyanamides R2R3NCN (R2/R3 = Alk/Alk, −(CH2)2O(CH2)2–, Ar/Ar, Ar/H) has been developed. In most cases, 2-amino-1,3-oxazoles functionalized at the nitrogen atom as well as at the fifth position of the heterocyclic ring (12 examples) were isolated in good to moderate yields.
Tetrahedron 2015, 71, 4616-4628
I.A. Smetanin, M.S. Novikov, N.V. Rostovskii, A.F. Khlebnikov, G.L. Starova, D.S. Yufit
“4-Halo-2-azabuta-1,3-dienes as intermediates in the rhodium carbenoid-initiated transformation of 2-halo-2H-azirines into 2,3-dihydroazetes and 2,5-dihydrooxazoles”
Tetrahedron, 2015, 71, 4616-4628
DOI:10.1016/j.tet.2015.05.022
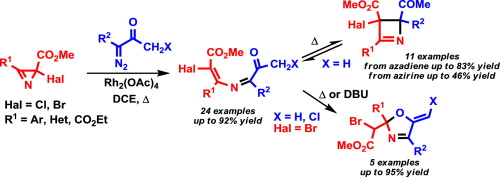
A wide range of electron-poor 4-bromo-/4-chloro-2-azabuta-1,3-dienes were synthesized by the Rh2(OAc)4-catalyzed reaction of diazo esters and diazo ketones with methyl 2-halo-2H-azirine-2-carboxylates. The E stereoselectivity with respect to the configuration of the Cdouble bond; length as m-dashC bond of the 2-azadiene moiety is in good agreement with the results of DFT (M06-2X/6-31+G(d,p)) calculations of the reaction pathway. The reaction proceeds via the formation of an azirinium ylide intermediate followed by ring opening with outward rotation of the halogen atom. Depending on the substitution pattern at C1 electron-poor 4-halo-2-azabutadienes can undergo two types of cyclization at elevated temperatures: reversible 1,4-electrocyclization to give 2,3-dihydroazetes or 1,5-exo-trig cyclization to give 5-methylene-2,5-dihydrooxazoles, both in good yields. The dihydrooxazole derivatives can be also obtained at ambient temperature under DBU catalysis.
Excursion for high school students

Excursion-lecturer was conducted in the Center for Magnetic Resonance for high school students from 403 schools.
Org. Biomolec. Chem. 2015, 13, 9825-9833
E.E. Galenko, O.A. Tomashenko, A.F. Khlebnikov, M.S. Novikov
“Metal/organo relay catalysis in a one-pot synthesis of methyl 4-aminopyrrole-2-carboxylates from 5-methoxyisoxazoles and pyridinium ylides”
Org. Biomolec. Chem., 2015, 13, 9825-9833
DOI:10.1039/c5ob01537e
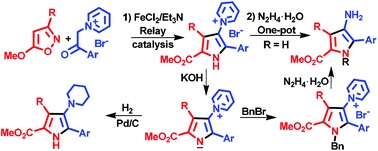
Methyl 4-aminopyrrole-2-carboxylates were synthesized in one-pot mode by the relay catalytic cascade reaction of 5-methoxyisoxazoles with pyridinium ylides by the use of a FeCl2/Et3N binary catalytic system leading to 1-(5-methoxycarbonyl-1H-pyrrol-3-yl)pyridinium salts followed by hydrazinolysis. The approach permits the introduction of a substituent at the pyrrole nitrogen via a nucleophilic reaction of the pyrrolylpyridinium ylide derived from the salt. Catalytic reduction of the ylides gives methyl 4-piperidinopyrrole-2-carboxylates.
Org. Lett. 2015, 17, 4148-4151
N.V. Rostovskii, P.A. Sakharo, M.S. Novikov, A.F. Khlebnikov, G.L. Starova
“Cu(I)-NHC-Catalyzed (2+3)-Annulation of Tetramic Acids with 2H-Azirines: Stereoselective Synthesis of Functionalized Hexahydropyrrolo[3,4-b]pyrroles”
Org. Lett., 2015, 17, 4148-4151
DOI:10.1021/acs.orglett.5b01883
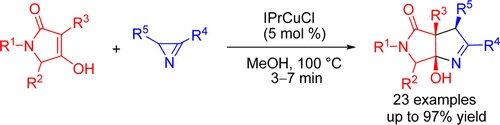
A stereoselective and high-yield synthesis of hexahydropyrrolo[3,4-b]pyrroles from tetramic acids and 2H-azirines under Cu(I)–NHC catalysis is developed. An unusual N–C2 azirine bond cleavage, initiated by a copper enolate, was rationalized in terms of a free radical reaction mechanism.

