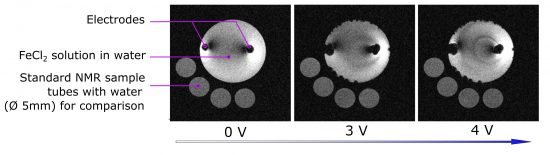
MRI of electrochemical cell with FeCl2 water solution under different applied voltage. Images were obtained on Bruker 400 WB Avance III spectrometer.
Archive for March 23, 2017
MRI view of electrochemical process
New J. Chem., 2017, 41, 316-325
Elena V. Andrusenko, Evgeniy V. Kabin, Alexander S. Novikov, Nadezhda A. Bokach, Galina L. Starova and Vadim Yu. Kukushkin
“Metal-mediated generation of triazapentadienate-terminated di- and trinuclear μ2-pyrazolate NiII species and control of their nuclearity”
New J. Chem., 2017, 41, 316-325
DOI:10.1039/C6NJ02962K
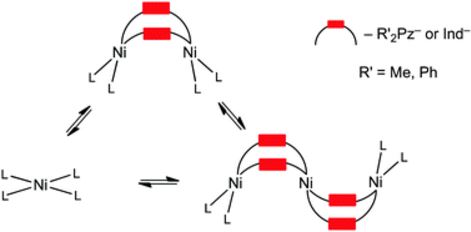
1,3,5-Triazapentadienate-terminated di- and trinuclear nickel(II) complexes featuring bridging azolate ligands, [Ni2(μ2-azolate)2(TAP)2] (TAP = H[N with combining low line][double bond, length as m-dash]C(OMe)NC(OMe)[double bond, length as m-dash][N with combining low line]H; azole = 3,5-Me2pyrazole 2, 3,5-Ph2pyrazole 3) and [Ni3(μ2-azolate)4(TAP)2] (azole = 3,5-Me2pyrazole 4, indazole 5), were obtained from systems Ni2+/NCNR2/azole systems in MeOH. The terminal TAP ligands in the [Ni2(μ2-azolate)2(TAP)2] and [Ni3(μ2-azolate)4(TAP)2] species originate from the previously unreported cascade NiII-mediated and chelation-driven reaction between cyanamides and methanol. The oligomeric species and also [Ni(TAP)2] (1) are subject to interconversions that depend on the reactants involved and the reaction conditions. The control of the nuclearity of the complexes can be achieved by changing the amount of azoles or by their protonation, alteration of the steric hindrance of the substituents in the heterocycles, and by changing the reaction temperature. Complexes 1–4 were characterized using elemental (C, H, N) analyses, 1H, 13C{1H} NMR, FTIR, HRESI-MS, TG-DTA, X-ray crystallography, and 5 was characterized using HRESI-MS and X-ray crystallography. Unconventional metallophilic contacts NiII⋯NiII were observed in dimer 3 in the solid state (the distance for Ni⋯Ni is 2.99 Å, whereas the double Bondi’s vdW radius for Ni is 3.26 Å) and the reality of these interactions was confirmed theoretically by the topological analysis of the electron density distribution (AIM method). The estimated energy for these non-covalent Ni⋯Ni interactions (ca. 4 kcal mol−1) fills the gap in the reported energies of the metal⋯metal interactions in a series comprising of NiII⋯NiII (this work), PdII⋯PdII (4.3–6.0 kcal mol−1), and PtII⋯PtII (3.9–11.7 kcal mol−1).
Inorganica Chimica Acta, 2017, 455, 607–612
Mikhail A. Kinzhalov, Galina L. Starova, Vadim P. Boyarskiy
“Interaction of benzene-1,2-diamines with isocyanide complexes of palladium(II): Insight into the mechanism”
Inorganica Chimica Acta, 2017, 455, 607–612
DOI:10.1016/j.ica.2016.05.014

Reactivity of 4-toluidine (2) and 4,5-dimethylbenzene-1,2-diamine (3) were compared in coupling with the palladium-bis(isocyanide) complex cis-[PdCl2(CNXyl)2] (Xyl = 2,6-Me2C6H3, 1), mixed isocyanide/diaminocarbene species cis-[PdCl2(CNXyl){C(NHXyl)double bond; length as m-dashNH(4-C6H4CH3)}] (4), and cis-[PdCl(CNXyl){C(NHXyl)double bond; length as m-dashNHC6H2(Me)2NH2}]Cl (5). In these Pd(II)-mediated reactions, 4,5-dimethylbenzene-1,2-diamine (3) was significantly more reactive than 4-toluidine (2), leading to the first mixed bis(diaminocarbene) complex cis-[PdCl{C(NHR)double bond; length as m-dashNHC6H2X2NH2}{C(NHXyl)double bond; length as m-dashNH(4-C6H4CH3)}]Cl (6) containing two different diaminocarbene ligands. Complex 6 was isolated and characterized by elemental analyses (C, H, N), HRESI+-MS, IR, 1H and 13C{1H} NMR spectroscopies, and single-crystal X-ray diffraction.
Excursion
![]() Students and lecturers from St. Petersburg State Technological Institute visited CMR. Mikhail Vovk has briefly demonstrated modern equipment of center and described its main application for users purposes.
Students and lecturers from St. Petersburg State Technological Institute visited CMR. Mikhail Vovk has briefly demonstrated modern equipment of center and described its main application for users purposes.