A.S. Pankova, P.R. Golubev, A.F. Khlebnikov, A.Yu. Ivanov, M.A. Kuznetsov
“Thiazol-4-one derivatives from the reaction of monosubstituted thioureas with maleimides: structures and factors determining the selectivity and tautomeric equilibrium in solution”
Beilstein J. Org. Chem., 2016, 12, 2563-2569
DOI:10.3762/bjoc.12.251
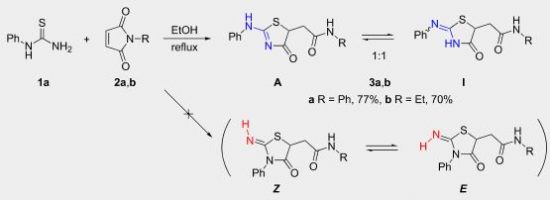
2-(Alkyl(aryl)amino)thiazol-4(5H)-ones can regioselectively be prepared from monoalkyl(aryl)thioureas and maleimides. In solution, the former heterocycles exist in a tautomeric equilibrium with 2-(alkyl(aryl)imino)thiazolidin-4-ones and the substituent on the exocyclic nitrogen atom governs the ratio of these tautomers. Isomers with the alkyl group in the endocyclic position can be obtained from N-methyl(ethyl)thioureas. 2D NMR spectroscopy and DFT calculations rationalize experimental results.