Total in December 1938 service applications were carried out.
All together measured:
- 1852 1H spectra
- 310 13C spectra
- 155 DEPT spectra
- 22 COSY spectra
- 15 NOESY spectra
- 36 31P spectra
- 28 19F spectra
212 applications were carried out.
Total in December 1938 service applications were carried out.
All together measured:
212 applications were carried out.
 Previously we have written about the PC program playing back FID data (see the post from 29.04.2015). There is a logical continuation of this idea: a whole Christmas song performed on NMR spectra of molecules, see also the original blog entry by Andrew Hall from the University of Bath.
Previously we have written about the PC program playing back FID data (see the post from 29.04.2015). There is a logical continuation of this idea: a whole Christmas song performed on NMR spectra of molecules, see also the original blog entry by Andrew Hall from the University of Bath.
M.A. Kuznetsov, A.N. Shestakov, M. Zibinsky, M. Krasavin, C.T. Supuran, S. Kalinin, M. Tanç
“Synthesis, structure and properties of N-aminosaccharin – A selective inhibitor of human carbonic anhydrase I”
Tetrahedron Letters, 2017, 58, 172–174
DOI:10.1016/j.tetlet.2016.12.005
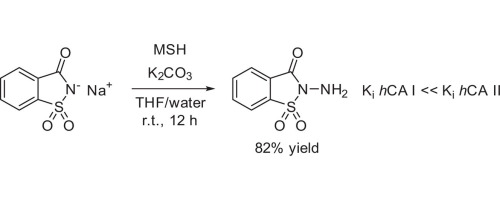
Previously unknown N-aminosaccharin was prepared in good yield via the one-step direct amination of saccharin sodium salt with hydroxylamine-O-mesitylenesulfonic acid (MSH) and its reactivity investigated. N-aminosaccharin and its derivatives were tested against hCA isoforms and the parent compound was identified to be a selective, low micromolar inhibitor (Ki = 8.8 lM) of hCA I. These findings provide a ligand-efficient starting point for the design of potent hCA I inhibitors – a promising drug target for retinal/cerebral edema treatment.
L. Sobczyk, D. Chudoba, P.M. Tolstoy, A. Filarowski
“Some Brief Notes on Theoretical and Experimental Investigations of Intramolecular Hydrogen Bonding”
Molecules, 2016, 21, 1657-1/19
DOI:10.3390/molecules21121657
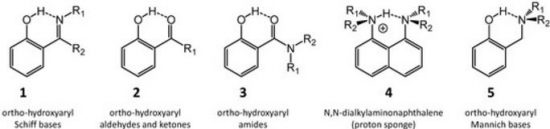
A review of selected literature data related to intramolecular hydrogen bonding in ortho-hydroxyaryl Schiff bases, ortho-hydroxyaryl ketones, ortho-hydroxyaryl amides, proton sponges and ortho-hydroxyaryl Mannich bases is presented. The paper reports on the application of experimental spectroscopic measurements (IR and NMR) and quantum-mechanical calculations for investigations of the proton transfer processes, the potential energy curves, tautomeric equilibrium, aromaticity etc. Finally, the equilibrium between the intra- and inter-molecular hydrogen bonds in amides is discussed.
B. Koeppe, S.A. Pylaeva, C. Allolio, D. Sebastiani, E.T.J. Nibbering, G.S. Denisov, H.-H. Limbach, P.M. Tolstoy
“Polar solvent fluctuations drive proton transfer in hydrogen bonded complexes of carboxylic acid with pyridines: NMR, IR and ab initio MD study”
Phys. Chem. Chem. Phys., 2016, accepted
DOI:10.1039/C6CP06677A
We study a series of intermolecular hydrogen-bonded 1 : 1 complexes formed by chloroacetic acid with 19 substituted pyridines and one aliphatic amine dissolved in CD2Cl2 at low temperature by 1H and 13C NMR and FTIR spectroscopy. The hydrogen bond geometries in these complexes vary from molecular (O–H…N) to zwitterionic (O…H–N+) ones, while NMR spectra show the formation of short strong hydrogen bonds in intermediate cases. Analysis of CQO stretching and asymmetric CO2 stretching bands in FTIR spectra
reveal the presence of proton tautomerism. On the basis of these data, we construct the overall proton transfer pathway. In addition to that, we also study by use of ab initio molecular dynamics the complex formed by chloroacetic acid with 2-methylpyridine, surrounded by 71 CD2Cl2 molecules, revealing a dualmaximum distribution of hydrogen bond geometries in solution. The analysis of the calculated trajectory shows that the proton jumps between molecular and zwitterionic forms are indeed driven by dipole–dipole solvent–solute interactions, but the primary cause of the jumps is the formation/breaking of weak CH…O bonds from solvent molecules to oxygen atoms of the carboxylate group.
Total in November 1963 service applications were carried out.
All together measured:
206 applications were carried out.
P. Golubev, E.A. Karpova, A.S. Pankova, M. Sorokina, M.A. Kuznetsov
“Regioselective Synthesis of 7‑(Trimethylsilylethynyl) pyrazolo [1,5‑a] pyrimidines via Reaction of Pyrazolamines with Enynones”
J. Org. Chem., 2016, 81, 11268-11275
DOI:10.1021/acs.joc.6b02217
Condensation of enynones readily available from cheap starting material with pyrazolamines provides easy access to fluorescent 7-(trimethylsilylethynyl)pyrazolo[1,5-a]pyrimidines. The reaction is straightforward, does not require the use of any additional reagents or catalysts, and can be performed without inert atmosphere. Various substituents and functional groups in both enynone and pyrazolamine are tolerated. The presented method features full regioselectivity, high isolated yields, and simplicity of both setup and product purification. Fluorescent properties of the obtained pyrazolopyrimidines were studied.
A.S. Pankova, P.R. Golubev, A.F. Khlebnikov, A.Yu. Ivanov, M.A. Kuznetsov
“Thiazol-4-one derivatives from the reaction of monosubstituted thioureas with maleimides: structures and factors determining the selectivity and tautomeric equilibrium in solution”
Beilstein J. Org. Chem., 2016, 12, 2563-2569
DOI:10.3762/bjoc.12.251
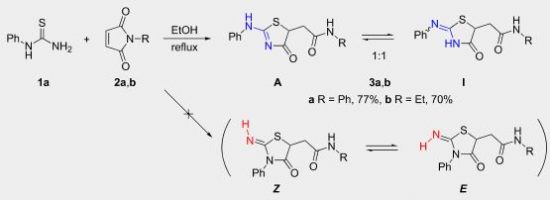
2-(Alkyl(aryl)amino)thiazol-4(5H)-ones can regioselectively be prepared from monoalkyl(aryl)thioureas and maleimides. In solution, the former heterocycles exist in a tautomeric equilibrium with 2-(alkyl(aryl)imino)thiazolidin-4-ones and the substituent on the exocyclic nitrogen atom governs the ratio of these tautomers. Isomers with the alkyl group in the endocyclic position can be obtained from N-methyl(ethyl)thioureas. 2D NMR spectroscopy and DFT calculations rationalize experimental results.