A.S. Mikherdov, M.A. Kinzhalov, A.S. Novikov, V.P. Boyarskiy, I.A. Boyarskaya, D.V. Dar’in, G.L. Starova, V.Yu. Kukushkin
“Difference in energy between two distinct types of chalcogen bonds drives regioisomerization of binuclear (Diaminocarbene)PdII complexes”
J. Am. Chem. Soc., 2016, 138, 14129-14137
DOI:10.1021/jacs.6b09133
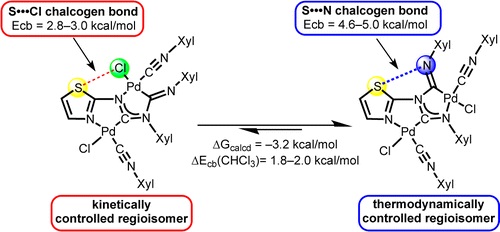
The reaction of cis-[PdCl2(CNXyl)2] (Xyl = 2,6-Me2C6H3) with various 1,3-thiazol- and 1,3,4-thiadiazol-2-amines in chloroform gives a mixture of two regioisomeric binuclear diaminocarbene complexes. For 1,3-thiazol-2-amines the isomeric ratio depends on the reaction conditions and kinetically (KRs) or thermodynamically (TRs) controlled regioisomers were obtained at room temperature and on heating, respectively. In CHCl3 solutions, the isomers are subject to reversible isomerization accompanied by the cleavage of Pd–N and C–N bonds in the carbene fragment XylNCN(R)Xyl. Results of DFT calculations followed by the topological analysis of the electron density distribution within the formalism of Bader’s theory (AIM method) reveal that in CHCl3 solution the relative stability of the regioisomers (ΔGexp = 1.2 kcal/mol; ΔGcalcd = 3.2 kcal/mol) is determined by the energy difference between two types of the intramolecular chalcogen bonds, viz. S···Cl in KRs (2.8–3.0 kcal/mol) and S···N in TRs (4.6–5.3 kcal/mol). In the case of the 1,3,4-thiadiazol-2-amines, the regioisomers are formed in approximately equal amounts and, accordingly, the energy difference between these species is only 0.1 kcal/mol in terms of ΔGexp (ΔGcalcd = 2.1 kcal/mol). The regioisomers were characterized by elemental analyses (C, H, N), HRESI+-MS and FTIR, 1D (1H, 13C{1H}) and 2D (1H,1H-COSY, 1H,1H-NOESY, 1H,13C-HSQC, 1H,13C-HMBC) NMR spectroscopies, and structures of six complexes (three KRs and three TRs) were elucidated by single-crystal X-ray diffraction.