Archive for October 31, 2016
Organometallics, 2016, 35, 3612-3623
D.S. Bolotin, A.S. Novikov, V.K. Burianova, M.Ya. Demakova, E.A. Daines, С. Pretorius, P. Mokolokolo, A. Roodt, N.A. Bokach, M.S. Avdontceva, A.P. Zhdanov, K.Yu. Zhizhin, N.T. Kuznetsov, V.Yu. Kukushkin
“Nucleophilicity of oximes based upon addition to а nitrilium closo-decaborate cluster”
Organometallics, 2016, 35, 3612-3623
DOI:10.1021/acs.organomet.6b00678
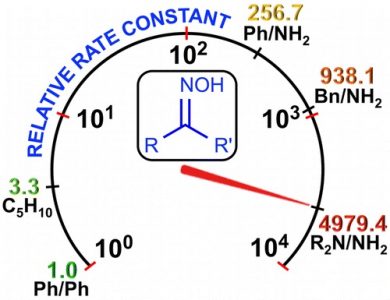
Three types of oxime species, i.e., 4-morpholylcarbamidoxime (hydroxyguanidine), phenylacetamidoxime and benzamidoxime (amidoximes), and cyclohexanone oxime and benzophenone oxime (ketoximes), react at room temperature with the 2-nitrilium closo-decaborate clusters, leading to 2-iminium closo-decaborates (14 examples; 57–94%). These species were characterized by ICPMS-based boron elemental analysis, HRESI–-MS, molar conductivity, IR, 1H{11B}, and 11B{1H} NMR spectroscopies, and additionally by single-crystal X-ray diffraction (for six compounds). On the basis of kinetic data, ΔH⧧, ΔS⧧, and ΔG⧧ of the additions were determined, showing a 4 order-of-magnitude decrease in reactivity from the hydroxyguanidine to the aromatic ketoxime as entering nucleophiles. The results of DFT calculations indicate that the mechanism for these reactions is stepwise and is realized through the formation of the orientation complex of the nitrone form, R2R3C═N+(H)O–, of oximes with [B10H9NCEt]−, giving further an acyclic intermediate (the rate-determining step), followed by proton migration, leading to the addition product. The calculated overall activation barrier for these transformations is consistent with the experimental kinetic observations. This work provides, for the first time, a broad nucleophilicity series of oximes, which is useful to control various nucleophilic additions of oxime species.
Excursion
Workshop in cmr
Our new colleagues have presented short talks about their areas of interest and scientific experience from previous education/work places:
- Roman Belykh “In-situ Analysis of nickel catalysts during CO2 methanation with FT-IR spectroscopy”
- Demidov Evgeniy “Surface active monomers application for carbon nanotubes – polymers composites preparation”
- Irina Alisova “Investigation suppression and generation mechanisms of triplet states for organic molecules”
Synthesis, 2016, 48, A-H
J.J. Medvedev, D.V. Semenok, X.V. Azarova, L.L. Rodina, V.A. Nikolaev
“A New Powerful Approach to Multi-Substituted 3(2H)-Furanones via Brønsted Acid-Catalyzed Reactions of 4-Diazodihydrofuran-3-ones”
Synthesis, 2016, 48, A-H
DOI:10.1055/s-0036-1588304
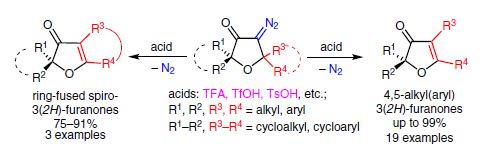
The interaction of 5,5-dialkyl(diaryl)-substituted 4-diazo-3(2H)furanones with Brønsted acids (TFA, TsOH, etc.) causes elimination of nitrogen accompanied by 1,2-nucleophilic rearrangement, giving rise to exclusive formation of 4,5-dialkyl(diaryl)-substituted 3(2Н)-furanones, ring-fused 3(2H)-furanones, and phenanthro[9,10-b]furan-3(2H)-ones in yields of up to 99%. The reaction is a new highly efficient way for the synthesis of multisubstituted 3(2Н)furanones.
Training course
Helvetica Chim. Acta, 2016, 99, 716-723
D.V. Semenok, J.J. Medvedev, M.S. Avdontceva, S.I. Selivanov, J. Sieler, A.S. Mereshchenko, V.A. Nikolaev
“Experimental Evidence of Intramolecular CAr–H···O=C Hydrogen Bonds in the Structure of (Diaryl)tetrahydrofuranones Using Spectroscopic Tools”
Helvetica Chim. Acta, 2016, 99, 716-723
DOI:10.1002/hlca.201600149
The occurrence of bifurcate H-bonds CAr–H···O=C in the structure of (diaryl)-tetrahydrofuranones was experimentally demonstrated using different methods and techniques. The consistent increasing spin–spin coupling constants 1J(C,H) of the ortho-H-atoms and low-field shift of v (C=O) in IR spectra of 2,2-(diaryl)tetrahydrofuran-3(2H)-ones relative to their 5,5-diaryl counterparts, as well as pronounced dependence of the ortho-C–H H-atoms chemical shifts on the temperature and solvent polarity along with X-ray diffraction analysis data unambiguously point to the existence of weak CAr–H···O=C H-bonds in these molecules.