D.V. Kurandina, E.V. Eliseenkov, T.S. Khaibulova, A.A. Petrov, V.P. Boyarskiy
“Copper-catalyzed C-N bond cross-coupling of aryl halides and amines in water in the presence of ligand derived oxalyl dihydrazide: scope and limitation”
Tetrahedron, 2015, 71, 7931-7937
DOI:10.1016/j.tet.2015.07.071
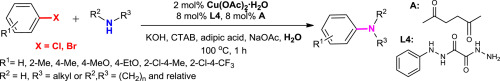
An efficient and convenient method has been developed for the copper-catalyzed C–N bond cross-coupling of aryl bromides with electron-donor substituents and aliphatic amines in water. The new ligand system N-phenyloxalyl bishydrazide/hexane-2,5-dione has been shown to be considerably more efficient in the copper-catalyzed C–N bond cross-coupling reaction as compared to the ligands described in the literature and allowed decreasing of the catalyst amount (up to 2 mol %) to achieve acceptable yields of isolated products (46–84%). Acceptor substituted aryl bromides, aryl bromides with substituents in the ortho-position, and some aryl dichlorides can undergo the C–N cross-coupling under the developed conditions, but their reactivity is lower.