Introductory NMR lecture for second year undergraduate students was carried out in Center for Magnetic Resonance.
Archive for November 13, 2015
Сontinuation of lectures
Lecture for the second year students
Excursion for school teachers
October
Total in October 1993 service applications were carried out.
All together measured:
- 1942 1H spectra
- 442 13C spectra
- 84 DEPT spectra
- 21 COSY spectra
- 24 NOESY spectra
- 39 31P spectra
- 83 19F spectra
138 applications were carried out.
J. Catalysis, 2015, 329, 449-456
S.A. Timofeeva, M.A. Kinzhalov, V.P. Boyarskiy, T.M. Buslaeva, E.A. Valishina, M. Haukka, K.V. Luzyanin, V.Yu. Kukushkin
“Application of palladium complexes bearing acyclic amino(hydrazido)carbene ligands as catalysts for copper-free Sonogashira cross-coupling”
J. Catalysis, 2015, 329, 449-456
DOI:10.1016/j.jcat.2015.06.001
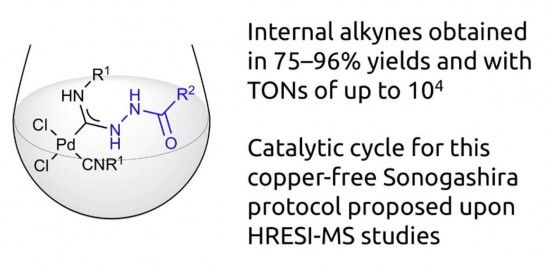
Metal-mediated coupling of one isocyanide in cis-[PdCl2(CNR1)2] (R1 = C6H11 (Cy) 1, tBu 2, 2,6-Me2C6H3 (Xyl) 3, 2-Cl-6-MeC6H34) and various carbohydrazides R2CONHNH2 [R2 = Ph 5, 4-ClC6H46, 3-NO2C6H47, 4-NO2C6H48, 4-CH3C6H49, 3,4-(MeO)2C6H310, naphth-1-yl 11, fur-2-yl 12, 4-NO2C6H4CH213, Cy 14, 1-(4-fluorophenyl)-5-oxopyrrolidine-3-yl 15, (pyrrolidin-1-yl)C(O) 16, 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propane-1-yl 17, EtNHC(O) 18] or sulfohydrazides R3SO2NHNH2 [R3 = Ph 19, 4-MeC6H420] led to a series of (hydrazido)(amino)carbene complexes cis-[PdCl2{C(NHNHX)double bond; length as m-dashN(H)R1}(CNR1)]; X = COR2, SO2R3 (21–48, isolated yields 60–96%). All prepared species were characterized by elemental analyses (C, H, N), HR ESI+-MS, IR, 1H and 13C{1H} NMR spectroscopy, and by a single-crystal X-ray diffraction for 38. Complexes 21–48 demonstrated excellent activity as catalysts in copper-free Sonogashira coupling of aryl iodides and a variety of aromatic terminal alkynes. Catalytic system runs in environmentally benign EtOH ensuring product yields of up to 75–96% and TONs of up to 104. Mechanism of the copper-free Sonogashira catalytic cycle involving 21–48 as catalysts was proposed upon identification of key intermediates using HRESI-mass.
Inorg. Chim. Acta 2015, 434, 31-36
A.A. Melekhova, A.S. Novikov, K.V. Luzyanin, N.A. Bokach, G.L. Starova, V.V. Gurzhiy, V.Yu. Kukushkin
“Tris-isocyanide copper(I) complexes: Synthetic, structural, and theoretical study”
Inorg. Chim. Acta, 2015, 434, 31-36
DOI:10.1016/j.ica.2015.05.002
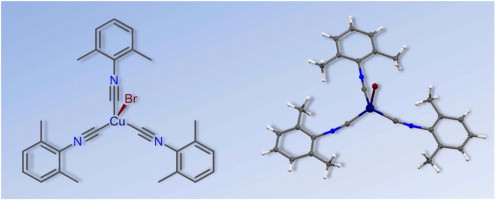
The reaction of CuBr with 3 equiv of CNR in CHCl3 at RT furnished the pure tris-isocyanide complexes [CuBr(CNR)3] [R = 2,6-Me2C6H3 (Xyl) (1), 2-Cl-6-MeC6H3 (2), 2-Naphthyl (3), C6H11 (Cy) (4)] in 85–99% isolated yield. Compounds 1–4 were characterized by elemental analyses (C, H, N), high resolution ESI+-MS, IR, 1H and 13C{1H} NMR spectroscopic techniques, and by single-crystal X-ray diffraction for 1 and 2. Coordination polyhedra of these two complexes are intermediate between tetrahedral and trigonal pyramidal with three isocyanide and one bromide ligands; the fragment M–C–N is bent [171.2(2)–175.6(2)°]. The influence of crystal packing effects on the geometric parameters of 1 as well as nature of the coordination Cu–C bonds and bond order of the formal triple C (triple bond)N bonds in this compound were also studied theoretically at the DFT level of theory. The crystal packing effects noticeably affect the values of the Cu–C–N angles and lead to a decrease of these angles approximately 10° relative to the gas phase geometries. Results of the AIM, NBO, and CDA analyses reveal that the Cu–C coordination bonds are of the electrostatic nature. Electrostatic interaction, unlike the covalent one, has no orientation, and it is the reason of the curved shape of the metal–ligand fragment.