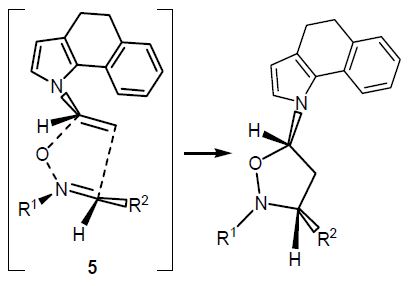
A.D. Lisakova, D.S. Ryabukhin, R.E. Trifonov, V.A. Ostrovskii, A.V. Vasilyev
“Alkylation of 5-substituted NH-tetrazoles by alcohols in the superacid CF3SO3H”
Tetrahedron Lett., 2015, 56, 7020-7023
DOI:10.1016/j.tetlet.2015.11.005
Reactions of 5-substituted NH-tetrazoles with alcohols in the superacid CF3SO3H have been studied. Both the structure of the tetrazole and the nature of alcohol were found to dramatically influence the selectivity of the reaction and yields of products. Tetrazoles bearing phenyl, electron-donating aryl, or benzyl groups at the 5-position, have been alkylated using various alcohols (including MeOH and EtOH) in CF3SO3H upon heating at 60 °C for 0.3–12 h to afford 2-alkyl-2H-tetrazoles in 30–98% yields.