D.I. Nilov, A.V. Vasilyev
“One-pot tandem hydrophenylation and ionic hydrogenation of 3-phenylpropynoic acid derivatives under superelectrophilic activation”
Tetrahedron Lett., 2015, 56, 5714-5717
DOI:10.1016/j.tetlet.2015.09.026
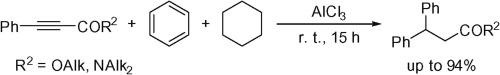
The reactions of esters and amides of 3-phenylpropynoic acid with strong Lewis acids AlX3 (X = Cl, Br) or conjugate Brønsted–Lewis superacids HX-AlX3 (X = Cl, Br) in benzene and cyclohexane at room temperature afforded 3,3-diphenylpropanoic acid derivatives in up to 94% yield. This tandem reaction of the acetylene bond proceeded by hydrophenylation followed by ionic hydrogenation.