A nice surprise for the Centers team was to see the publication in collaboration with the staff on the front cover of “Journal of Physical Chemistry B”.
S.O. Rabdano, A.V. Donets, M.A. Vovk, D.Michel, V.I. Chizhik
“ “Hydration Shells” of CH2 Groups of ω‑Amino Acids as Studied by Deuteron NMR Relaxation”
J. Phys. Chem. B, 2015, 119, 13358-13366
DOI:10.1021/acs.jpcb.5b06584
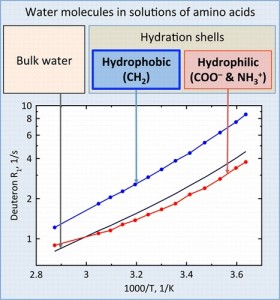
Hydration phenomena play a very important role in various processes, in particular in biological systems. Water molecules in aqueous solutions of organic compounds can be distributed among the following substructures: (i) hydration shells of hydrophilic functional groups of molecules, (ii) water in the environment of nonpolar moieties, and (iii) bulk water. Up to now, the values of hydration parameters suggested for the description of various solutions of organic compounds were not thoroughly analyzed in the aspect of the consideration of the total molecular composition. The temperature and concentration dependences of relaxation rates of water deuterons were studied in a wide range of concentration and temperature in aqueous (D2O) solutions of a set of ω-amino acids.
Assuming the coordination number of the CH2 group equal to 7, which was determined from quantum-chemical calculations, it was found that the rotational correlation times of water molecules near the methylene group is 1.5−2 times greater than one for pure water. The average rotational mobility of water molecules in the hydration shells of hydrophilic groups of ω-amino acids is a bit slower than that in pure solvent at temperatures higher that 60 °C, but at lower temperatures, it is 0.8−1.0 of values of correlation times for bulk water. The technique suggested provides the basis
for the characterization of different hydrophobic and hydrophilic species in the convenient terms of the rotational correlation times for the nearest water molecules.