New employees of Center for Magnetic Resonance Ivan Giba and Katharine Grebenyuk have briefly presented they research iterests.
Archive for September 30, 2015
Seminar, Ivan Giba and Katharine Grebenyuk
Guest from Puebla

Carlos Ernesto Avila Crisostomo from Institute for Physics “Ing. Luis Rivera Terrazas” (Puebla, Mexico) investigates magnetic properties of nanostructures based on synthetic opals.
Guests from INEOS RUS

Gleb A. Silantyev from the Laboratory for Metal Hydrides (A.N. Nesmeyanov Institute of Organoelement Compounds Russian Academy of Sciences) investigates dihydrogen bonds of transition metal hydrides.
Dalton Trans. 2015, accepted
A. S. Antonov, A. F. Pozharskii, V. A. Ozeryanskii, A. Filarowski,
K. Yu. Suponitsky, P. M. Tolstoy, M. A. Vovk
“Ring Lithiation of 1,8-Bis(dimethylamino)naphthalene: Another Side of the ‘Proton Sponge Coin’”
Dalton Trans., 2015, accepted
DOI:10.1039/C5DT02482J
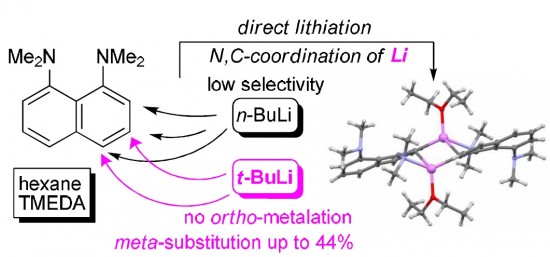
It has been found that 1,8-bis(dimethylamino)naphthalene (DMAN), unlike N,N-dimethylaniline, undergoes ring metallation in n-BuLi–TMEDA–Et2O system with low selectivity and in poor total yield. The situation is significantly improved in t-BuLi–TMEDA–n-hexane system when 3- and 4-lithium derivatives become the only reaction products in good yield. The formation of 3-Li-DMAN is especially fortunate since no method of direct meta-functionalization of DMAN has been known to date. The relative stability and structure of DMAN lithium derivatives have been examined with the help of X-ray and multinuclear NMR measurements as well as DFT calculations.
Tetrahedron Lett. 2015, 56, 5381-5385
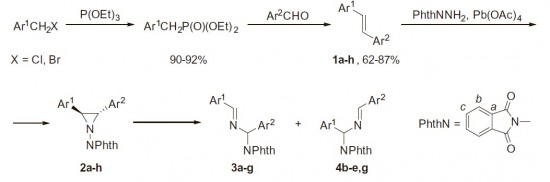
A.S. Pankova, M.V. Sorokina, М.А. Кuznetsov.
“Thermal rearrangement of 2,3-diaryl-1-phthalimidoaziridines”
Tetrahedron Lett., 2015, 56,5381-5385
DOI:10.1016/j.tetlet.2015.07.093
2,3-Diaryl-1-phthalimidoaziridines and 2,3-diaryl-1-phthalimidoaziridine-2-carbonitriles were found to readily undergo thermal rearrangement into imines via 1,2-migration of the phthalimido group and accompanying C–C bond cleavage. Isomerization proceeds regioselectively with preferable migration to the electron-deficient carbon atom. Interestingly, this reaction was found to predominate even in the presence of dipolarophiles.
Reac. Kinet. Mech. Cat. 2015, 116, 43-50
S. Lednev, A. Sirick, E. Pliss, A. Rusakov, N. Shvyrkova, A. Ivanov.
“Influence of solvation on the kinetics of methyl methacrylate oxidation inhibited by aromatic amines”
Reac. Kinet. Mech. Cat., 2015, 116,43-50
DOI:10.1007/s11144-015-0881-9
The kinetics of methyl methacrylate oxidation inhibited by aromatic amines in media of different polarities was studied. The complexing parameters in systems “oxidation substrate—aromatic amine—solvent” using IR and NMR spectroscopy were found. The correlations between inhibited oxidation rate constant and the polarity of the medium were obtained using Kirkwood–Onsager equation.
Lecture of Andreas Roodt
On September 22th Andreas Roodt (Department of Chemistry, University of the Free State, Bloemfontein 9300, South Africa) will present a lecture “Are Detailed Reaction Mechanisms Really Necessary in (Applied) Organometallic and Coordination Chemistry?”. The seminar will take place at 17:15 at the Institute of Chemistry in the audience 1093.
August
Total in August 716 service applications were carried out.
All together measured:
- 686 1H spectra
- 162 13C spectra
- 91 DEPT spectra
- 10 COSY spectra
- 19 NOESY spectra
- 41 31P spectra
- 44 19F spectra
100 applications were carried out.