E.Yu. Tupikina, G.S. Denisov, P.M. Tolstoy
“NMR Study of CHN Hydrogen Bond and Proton Transfer in 1,1-dinitroethane Complex with 2,4,6-trimethylpyridine”
J. Phys. Chem. A., 2014, accepted
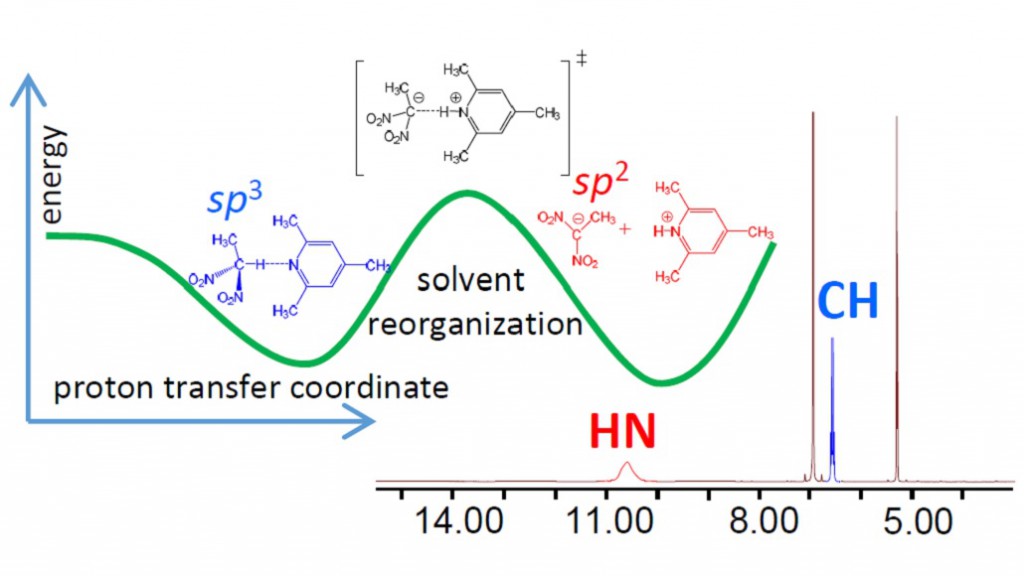
Intermolecular complex with CHN hydrogen bond formed by 1,1-dinitroethane (DNE) and 2,4,6-trimethylpyridine (collidine) dissolved in CD2Cl2 was studied experimentally by 1H NMR spectroscopy at 180-300 K. Equilibrium between molecular CH⋅⋅⋅N form and zwitterionic C−/HN+ form was detected in slow exchange regime in NMR time scale. No sign of direct C−⋅⋅⋅HN+ bond was observed; the ion pair is likely to be held by Coulomb interactions. Moreover, there are indications that the protonated base is involved in formation of homo-conjugated (NHN)+ collidine-collidinium hydrogen bonded complexes.
The reaction pathway of proton transfer in DNE-pyiridine complex in vacuum was studied computationally at B3LYP/6-31++G(d,p) level of theory. NMR chemical shifts and coupling constants were calculated for a series of snapshots along the proton transfer coordinate. While central carbon atom has pyramidal (sp3) configuration in DNE, it is flat (sp2) in DNE carbanion. As a result, the most indicative computed NMR parameter reflecting hybridization of carbon atom appeared to be 1JCC, which starts to change rapidly as soon as structure with quasi-symmetric C⋅⋅H⋅⋅N bond is reached. Couplings within the hydrogen bridge, 1JCH, 1hJHN and 2JCN, can serve as good indicators of the degree of proton transfer.